��Ŀ����
��֪��25����������10.00mL0.1mol?L-1HCOOH��Һ����μ���0.1mol?L-1NaOH��Һ����pH�仯������ͼ��ʾ�������¶ȱ仯��������˵���в���ȷ���ǣ�������
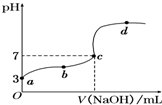
| A��a���ʾ����Һ��c��HCOO-��ԼΪ10-3mol?L-1 |
| B����25mL����ʽ�ζ�����ȡHCOOH��Һ |
| C��c��NaOH��Һ�����С��10mL |
| D����a��c����һ�㣬��Һ��һ������c��Na+����c��HCOO-����c��H+����c��OH-�� |

A�����ڼ�����ˮ�����������Ũ�Ⱥ�С��������Һ��������Ũ����������ļ��������Ũ�Ȼ�����ȣ�a��ʱ��ҺpH=3��������Ũ��Ϊ10-3mol?L-1���������Һ�м��������Ũ��ԼΪ10-3mol?L-1����A��ȷ��
B������ΪһԪ���ᣬ��Һ��ʾ���ԣ�����ȡ�ü���Ӧ��ʹ����ʽ�ζ��ܣ���B��ȷ��
C�����ڼ���Ϊ���ᣬ������10mL����������Һʱ����Ӧ����ǿ�������μ����ƣ���Ӧ�����ҺΪ���ԣ�����c����ʾ���ԣ�����������������Һ���Ӧ��С��10mL����C��ȷ��
D����a��c����һ�㣬��Һ��ʾ���ԣ���һ�����㣺c��H+����c��OH-�������ݵ���غ㣺c��Na+��+c��H+��=c��HCOO-��+c��OH-����֪��c��Na+����c��HCOO-������������Ũ����ȷ��ϵΪ��c��HCOO-����c��Na+����c��H+����c��OH-������D����
��ѡD��
B������ΪһԪ���ᣬ��Һ��ʾ���ԣ�����ȡ�ü���Ӧ��ʹ����ʽ�ζ��ܣ���B��ȷ��
C�����ڼ���Ϊ���ᣬ������10mL����������Һʱ����Ӧ����ǿ�������μ����ƣ���Ӧ�����ҺΪ���ԣ�����c����ʾ���ԣ�����������������Һ���Ӧ��С��10mL����C��ȷ��
D����a��c����һ�㣬��Һ��ʾ���ԣ���һ�����㣺c��H+����c��OH-�������ݵ���غ㣺c��Na+��+c��H+��=c��HCOO-��+c��OH-����֪��c��Na+����c��HCOO-������������Ũ����ȷ��ϵΪ��c��HCOO-����c��Na+����c��H+����c��OH-������D����
��ѡD��

��ϰ��ϵ�д�
�����Ŀ