��Ŀ����
����ѪҺ��Ca2+��Ũ��һ�����g/cm3����ʾ(��1 cm3Ѫ���к��е�Ca2+������)����ȡ һ�������Ѫ�����������IJ���泥�(NH4)2C2O4����Һ�������������(CaC2O4)���������˲���Ƴ���ϴ�Ӻ�����ǿ��ɵ��������(H2C2O4)������KMnO4��Һ�ζ����ɲⶨѪҺ��Ʒ��Ca2+��Ũ�ȡ�ij�о���ѧϰС���������ʵ�鲽��ⶨѪҺ��Ʒ��Ca2+��Ũ�ȡ�
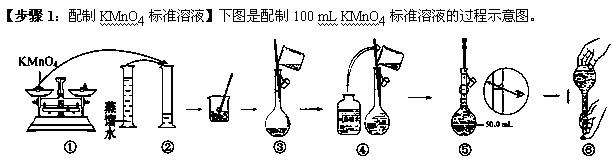 (1)����۲�ͼʾ�жϣ����в���ȷ����������� (�����)��
(1)����۲�ͼʾ�жϣ����в���ȷ����������� (�����)��
(2)����ȷ��100 mL��Һ�����������__________________
(3)�����ͼʾ�IJ��������Ƶ���Һ����ʵ�飬������������ȷ������£������Ƶ���ҺŨ�Ƚ�
______(�ƫ��ƫС��)��
������2���ⶨѪҺ��Ʒ��Ca2+��Ũ�ȡ���ȡѪ��20.00 mL����������������õ����ᣬ����0.020 mol/L ����KMnO4��Һ�ζ���ʹ����ת����CO2�ݳ�����ʱ������12.00 mL KMnO4��Һ��
(4)д������������KMnO4��Һ��Ӧ�����ӷ���ʽ ��
(5)�ζ����յ�Ϊ ��
(6)�������㣬ѪҺ��Ʒ��Ca2+��Ũ��Ϊ__________g/cm3��
(1) �ڢݣ�2�֣� (2) 100 mL����ƿ��2�֣� (3)ƫС ��2�֣�
(4) 2MnO4-+5H2C2O4+6H+ == 2 Mn2++10CO2��+8H2O ��2�֣�
(5)�������һ��KMnO4��Һʱ����Һ����ɫ��Ϊ�Ϻ�ɫ���Ұ�����ڲ���ɫ����2�֣�
��6��1.2��10-3��2�֣�

 �Ͻ�ƽСѧ��������ϵ�д�
�Ͻ�ƽСѧ��������ϵ�д� �Ƹ������������ϵ�д�
�Ƹ������������ϵ�д�