��Ŀ����
����Ŀ�������Ȼ�ѧ����ʽ�еķ�Ӧ�ȣ���ʾȼ���ȵ��ǣ� ��
A. NH3(g)+![]() O2(g)
O2(g)![]()
![]() N2(g)+
N2(g)+![]() H2O(g) ��H=-a kJ��mol-1
H2O(g) ��H=-a kJ��mol-1
B. C6H12O6(s)+6O2(g)![]() 6CO2(g)+6H2O(l) ��H=-b kJ��mol-1
6CO2(g)+6H2O(l) ��H=-b kJ��mol-1
C. C(s) +H2O(g) == CO(g)+H2(g) H=-c kJ��mol-1
D. CH3CH2OH(l)+![]() O2(g)
O2(g)![]() CH3CHO(l)+H2O(l) ��H=-d kJ��mol-1
CH3CHO(l)+H2O(l) ��H=-d kJ��mol-1
���𰸡�B
��������
A. NH3(g)+![]() O2(g)
O2(g)![]()
![]() N2(g)+
N2(g)+![]() H2O(g) ��H=-a kJ��mol-1��ˮ�����壬���ܱ�ʾȼ���ȣ��ʲ�ѡA��
H2O(g) ��H=-a kJ��mol-1��ˮ�����壬���ܱ�ʾȼ���ȣ��ʲ�ѡA��
B. C6H12O6(s)+6O2(g)![]() 6CO2(g)+6H2O(l) ��H=-b kJ��mol-1�����ɶ�����̼�����Һ̬ˮ���ܱ�ʾȼ���ȣ���ѡB��
6CO2(g)+6H2O(l) ��H=-b kJ��mol-1�����ɶ�����̼�����Һ̬ˮ���ܱ�ʾȼ���ȣ���ѡB��
C. C(s) +H2O(g) =CO(g)+H2(g) ��H=-c kJ��mol-1���������ɶ�����̼�����Һ̬ˮ�����ܱ�ʾȼ���ȣ��ʲ�ѡC��
D. CH3CH2OH(l)+![]() O2(g)
O2(g)![]() CH3CHO(l)+H2O(l) ��H=-d kJ��mol-1���������ɶ�����̼�����Һ̬ˮ�����ܱ�ʾȼ���ȣ��ʲ�ѡD��
CH3CHO(l)+H2O(l) ��H=-d kJ��mol-1���������ɶ�����̼�����Һ̬ˮ�����ܱ�ʾȼ���ȣ��ʲ�ѡD��
�����ѡB

 �¿α�����Ķ�ѵ��ϵ�д�
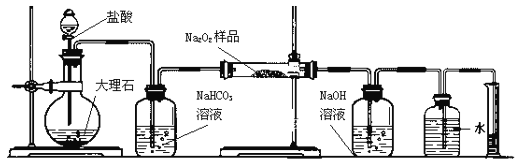
�¿α�����Ķ�ѵ��ϵ�д�����Ŀ���������Ʊ��治�����ױ��ʡ�ij����С��Ϊ�˴��Բⶨ�������Ƶ��������������dz�ȡ2.0g��Ʒ�����������ͼװ�����ⶨ�������Ƶ�����������ͼ�е�E��F��������װ�ã������ⶨO2�������
| |||||
A | B | C | D | E | F |
(1)д��װ��A��������Ҫ��������������__________��_______��
(2)д��װ��A�з��������ӷ���ʽ____________________________��
(3)װ��B��������______________________________��
(4)д��װ��C�з�����Ӧ����Ҫ��ѧ����ʽ��______________________________��
(5)װ��D�� NaOH��������________________________________________��
(6)�����ڶ�����Ͳ��ˮ�������������ɱ�״�������������Ϊ224mL������Ʒ�й������Ƶ���������Ϊ__________