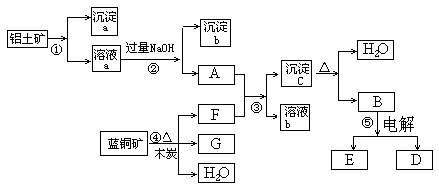
��Ŀ����
��14�֣���֪�������������������ԭ��Ӧ��ʱ��һ������Ũ��Խϡ����Ӧ�Ļ�ԭ�����е��Ļ��ϼ�Խ�͡�����һ�������������Ͻ���һ����ϡHNO3��ַ�Ӧ����Ӧ���������κ�����ų����ڷ�Ӧ���������Һ�У���μ���4 mol/L NaOH��Һ������NaOH��Һ�������V����������������ʵ�����n����ϵ��ͼ��ʾ����
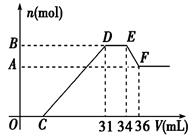
��1����д��DE�Ρ�EF����������Ӧ�����ӷ� ��ʽ��
��ʽ��
DE��
EF��
��2���Ͻ����������ʵ���Ϊ mol
��3���Ͻ��н��������ʵ�����Ϊ mol
��4�����C���ֵΪ ml

��1����д��DE�Ρ�EF����������Ӧ�����ӷ�
 ��ʽ��
��ʽ��DE��
EF��
��2���Ͻ����������ʵ���Ϊ mol
��3���Ͻ��н��������ʵ�����Ϊ mol
��4�����C���ֵΪ ml
��1�� 
 ����2�֣�
����2�֣�
��2��0.008mol��3�֣��� ��3��0.032mol��3�֣� ��4��7ml��4�֣�

 ����2�֣�
����2�֣���2��0.008mol��3�֣��� ��3��0.032mol��3�֣� ��4��7ml��4�֣�
��

��ϰ��ϵ�д�
 ��ĩ���100�ִ��½����ȫ�Ծ�ϵ�д�
��ĩ���100�ִ��½����ȫ�Ծ�ϵ�д�
�����Ŀ
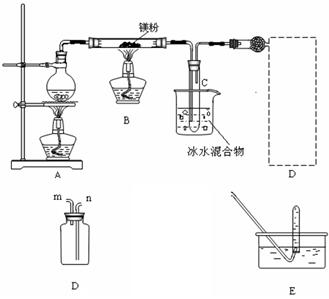
 �ȹ۲쵽���������ٵ�ȼ��һ�ƾ��ơ���������Ŀ������������������
�ȹ۲쵽���������ٵ�ȼ��һ�ƾ��ơ���������Ŀ������������������ Ӧһ��ʱ���Bװ�ò������п��ܲ����Ĺ������ʳ�MgO�⣬������M
Ӧһ��ʱ���Bװ�ò������п��ܲ����Ĺ������ʳ�MgO�⣬������M g��Mg(OH)2 ��ѡ����һ�֣����ʵ��֤�����Ĵ��ڣ���Ҫд����Ҫ�����������Լ��������ۡ�
g��Mg(OH)2 ��ѡ����һ�֣����ʵ��֤�����Ĵ��ڣ���Ҫд����Ҫ�����������Լ��������ۡ�