��Ŀ����
��9�֣���ˮ�Ȼ����ǰ�ɫ���壬������ˮ�֣���178��������װ����ˮ�Ȼ������Լ�ƿ�����ڳ�ʪ�����У����Զ���ը�����������������Ȼ�������Ϊ�л��ϳɺ�ʯ��ҵ�Ĵ����������ڴ������͵ȡ���ҵ���ɽ��������������û�����ˮ�Ȼ������������ڽ����������Ƶá�






ij������ȤС����ʵ�����У�ͨ����ͼװ����ȡ������������ˮ�Ȼ�����

��1��Aװ���з�����Ӧ�Ļ�ѧ����ʽΪ ��
��2��B��Cװ����Ӧʢ�ŵ��Լ����Ʒֱ�Ϊ �� ��
��3����Aװ�õ�����������������B��Cװ�ö�ֱ�ӽ���D�ܣ�����ʵ������IJ�������� ��
��4��Fװ������������� ��







ij������ȤС����ʵ�����У�ͨ����ͼװ����ȡ������������ˮ�Ȼ�����

��1��Aװ���з�����Ӧ�Ļ�ѧ����ʽΪ ��
��2��B��Cװ����Ӧʢ�ŵ��Լ����Ʒֱ�Ϊ �� ��
��3����Aװ�õ�����������������B��Cװ�ö�ֱ�ӽ���D�ܣ�����ʵ������IJ�������� ��
��4��Fװ������������� ��
��1��
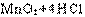 (Ũ)
(Ũ)
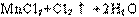 ��2�֣�
��2�֣���2������ʳ��ˮ��Ũ���ᣨÿ��1�֣���2�֣�
��3��δ����ȥ��HCl��ˮ������Cl2����D �У�1�֣�����Al��Ӧ������H2��1�֣���H2��Cl2 ��ϻᷢ����ը��1�֣�
��4�����ն������������ֹ��Ⱦ��������ֹ�����е�ˮ��������D�У�2�֣���
��

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
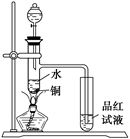
 2��ϡ���Ṳ������ȡ����
2��ϡ���Ṳ������ȡ����