��Ŀ����
12.0 gijҺ̬�л�������A��ȫȼ�պ�����14.4 g H2O��26.4 gCO2������л�������A��������H2������ܶ���30����

��1���л���A�ķ���ʽ ��
��2������ͼ��ʾװ�òⶨ�л���A�ķ��ӽṹ��ʵ���������£�ʵ�����ݾ��ѻ���Ϊ��״������a mL���ܶ�Ϊ �����л���A����������ȫ��Ӧ����ͲҺ�����Ϊb mL����1 molA��������x mol��ԭ���ܸ������Ʒ�Ӧ����x�ļ���ʽΪ �����Բ�����
�����л���A����������ȫ��Ӧ����ͲҺ�����Ϊb mL����1 molA��������x mol��ԭ���ܸ������Ʒ�Ӧ����x�ļ���ʽΪ �����Բ�����

��1���л���A�ķ���ʽ ��
��2������ͼ��ʾװ�òⶨ�л���A�ķ��ӽṹ��ʵ���������£�ʵ�����ݾ��ѻ���Ϊ��״������a mL���ܶ�Ϊ
 �����л���A����������ȫ��Ӧ����ͲҺ�����Ϊb mL����1 molA��������x mol��ԭ���ܸ������Ʒ�Ӧ����x�ļ���ʽΪ �����Բ�����
�����л���A����������ȫ��Ӧ����ͲҺ�����Ϊb mL����1 molA��������x mol��ԭ���ܸ������Ʒ�Ӧ����x�ļ���ʽΪ �����Բ�������1��C3H8O��2�֣�
��2��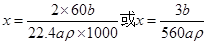 ���������ֻ���ı���ʽ���ɵ÷֣���2�֣�
���������ֻ���ı���ʽ���ɵ÷֣���2�֣�
��2��
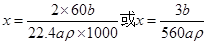 ���������ֻ���ı���ʽ���ɵ÷֣���2�֣�
���������ֻ���ı���ʽ���ɵ÷֣���2�֣������������1�����ݰ����ӵ����ɿ�֪������ͬ�������������Է�������֮�ȵ����ܶ�֮�ȣ��û����������������������ܶ�Ϊ30����û��������Է�������Ϊ30��2��60����12g�л�������ʵ�����
12g��60g/mol��0.2mol
n��H2O����14.4g��18/mol��0.8mol
n��CO2����26.4g��44/mol��0.6mol
����12.0g������m��C����0.6mol��12g/mol��7.2g
m��H����2��0.8mol��1g/mol��1.6g
��m��O������12.0g��7.2g��1.6g��3.2g
n��O����3.2g��16g/mol��0.2mol
����������N��C����3��N��H����8��N��O����1
���л������ʽΪC3H8O
��2��amL���ܶ�Ϊ��g/cm3�����л���A������Ϊ��m��a��g
���ʵ���Ϊ��a��g��60g/mol��
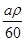 mol
mol��ͲҺ�����Ϊbml�����������������Ϊbml
��n��H2����0.001bL��22.4L/mol
��C3H8O+xNa
 C3H��8-x��ONa+0.5xH2
C3H��8-x��ONa+0.5xH21 0.5x
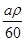 mol
mol 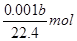
��֮��
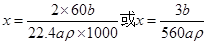
�����������ǻ���������Ŀ��飬���������̲ģ����ۻ����������ڵ���ѧ����ѧϰ��Ȥ��ѧϰ�����ԣ��ѶȲ�����Ĺؼ��Ǹ���ԭ���غ㷨���������������ѧ���Ĺ淶����������

��ϰ��ϵ�д�
�����Ŀ
 2HNO3+4N2��+9H2O�У�ÿ����4molN2��ת�Ƶ�����Ϊ15NA
2HNO3+4N2��+9H2O�У�ÿ����4molN2��ת�Ƶ�����Ϊ15NA
