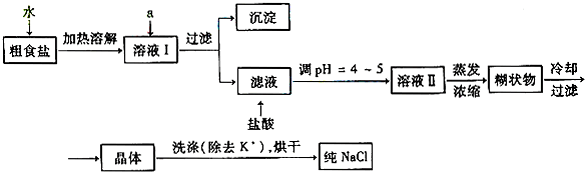
��Ŀ����
ʳ�����ճ�����ı���Ʒ��Ҳ����Ҫ�Ļ���ԭ�ϡ�
��1����ʳ�γ���������K+��Ca2+��Mg2+��Fe3+��SO42�����������ӣ�ʵ�����ᴿNaCl���������£�
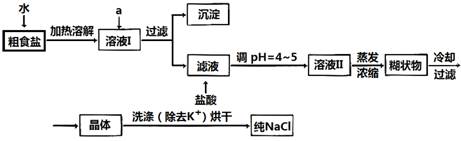
�ṩ���Լ�������Na2CO3��Һ ����K2CO3��Һ NaOH��Һ BaCl2��Һ
Ba��NO3��2��Һ 75%�Ҵ������Ȼ�̼
������ȥ��ҺI�е�Ca2+��Mg2+��Fe3+��SO42�����ӣ�ѡ��a���������Լ������μ�˳������Ϊ_______ __��ֻ�ѧʽ����
��ϴ�ӳ�ȥNaCl������渽��������KCl��ѡ�õ��Լ�Ϊ_____ ____��
 ��2�����ᴿ��NaCl����500 mL4��00
mol��L-1NaCl��Һ��
��2�����ᴿ��NaCl����500 mL4��00
mol��L-1NaCl��Һ��
������������ƽ��ҩ�ס����������___ _____
�����������ƣ���
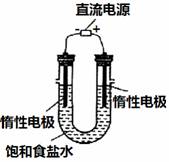
��3����ⱥ��ʳ��ˮ��װ����ͼ��ʾ�����ռ���H2Ϊ2 L��
��ͬ���������ռ���Cl2_____���>������=����<����2 L��
ԭ����_________��װ�øĽ��������Ʊ�NaOH��Һ��
���ⶨ��Һ��NaOH��Ũ�ȣ����õķ�����__________��
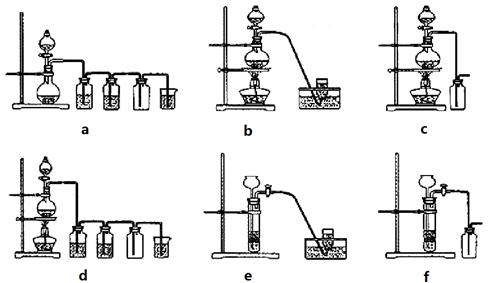
��4��ʵ�����Ʊ�H2��Cl2ͨ���������з�Ӧ��
Zn+H2SO4
ZnSO4+H2����MnO2+4HCl��Ũ�� MnCl2+Cl2��+2H2O
MnCl2+Cl2��+2H2O
�ݴˣ���������������װ����ѡ���Ʊ����ռ�H2��װ��_______������ţ����Ʊ����ռ��������Cl2��װ��_________������ţ���
��ѡ���Ʊ������װ�ã�
��1����BaCl2��NaOH��Na2CO3��2�֣�����75���Ҵ���2�֣���
��2���ձ���500 mL����ƿ����ͷ�ιܣ�2�֣���
��3��< ��1�֣���������ɵ������������ɵ�NaOH�����˷�Ӧ��2�֣���
�к͵ζ���2�֣�
��4��e��2�֣���d��2�֣���