��Ŀ����
C��Si�ֱ��ǹ������������ʯ����ҪԪ�أ�
��1��CH4��SiH4�е�ϸߵ��� ��ԭ���� ���ȶ��Խϸߵ��� ��
��2��SiC�ľ���ṹ�뾧������ƣ�����Cԭ�ӵ��ӻ���ʽΪ ��������ڵ��������� ��
��3��CO2�γɵĸɱ�������SiO2������ȣ�����˵������ȷ���� ��
A������ԭ�ӵ��ӻ���ʽ��ͬ
B�����ɾ���������ͬ
C��������������������ͬ
D�����嵼���Բ�ͬ
E������ʱ������ӷ��Բ�ͬ
��4����֪�����������£���ѧ��Ӧ�ġ�H���ڷ�Ӧ�ж��Ѿɻ�ѧ���ļ���֮���뷴Ӧ���γ��»�ѧ���ļ���֮�͵IJд��Si��s����O2��ȼ������SiO2��s�����Ȼ�ѧ����ʽ ��
��5���������ǹ��ɵ����ʵ����ʻ������ʰ���H2N-CH2-COOH�У���Ԫ�صĵ縺�ԴӴ�СΪ ��
��1��CH4��SiH4�е�ϸߵ���
��2��SiC�ľ���ṹ�뾧������ƣ�����Cԭ�ӵ��ӻ���ʽΪ
��3��CO2�γɵĸɱ�������SiO2������ȣ�����˵������ȷ����
A������ԭ�ӵ��ӻ���ʽ��ͬ
B�����ɾ���������ͬ
C��������������������ͬ
D�����嵼���Բ�ͬ
E������ʱ������ӷ��Բ�ͬ
��4����֪�����������£���ѧ��Ӧ�ġ�H���ڷ�Ӧ�ж��Ѿɻ�ѧ���ļ���֮���뷴Ӧ���γ��»�ѧ���ļ���֮�͵IJд��Si��s����O2��ȼ������SiO2��s�����Ȼ�ѧ����ʽ
| ��ѧ�� | Si-O | O=O | Si-Si |
| ���ܣ�kJ?mol-1�� | 460 | 498 | 226 |
���㣺����������������۵㡢Ӳ�ȡ������ԵȵĹ�ϵ,�Ȼ�ѧ����ʽ
ר�⣺��ѧ��Ӧ�е������仯,��ѧ���뾧��ṹ
��������1��������Է��������жϣ��ǽ�����Խǿ�����⻯��Խ�ȶ���
��2�����ݼ۲���Ӷ����жϣ��ǽ���ԭ�Ӽ����γɹ��ۼ���
��3���ɱ����ڷ��Ӿ��壬������������ԭ�Ӿ��壬���ݾ���Ĺ�����������
��4�����ݷ�Ӧ�ʱ��H=��Ӧ������ܺ�-����������ܺ����������㣻
��5������Ԫ�صķǽ������жϣ��ǽ�����Խǿ����縺��Խǿ��
��2�����ݼ۲���Ӷ����жϣ��ǽ���ԭ�Ӽ����γɹ��ۼ���
��3���ɱ����ڷ��Ӿ��壬������������ԭ�Ӿ��壬���ݾ���Ĺ�����������
��4�����ݷ�Ӧ�ʱ��H=��Ӧ������ܺ�-����������ܺ����������㣻
��5������Ԫ�صķǽ������жϣ��ǽ�����Խǿ����縺��Խǿ��
���
�⣺��1������ͬ����Ԫ���γɵ��⻯���ɽṹ���ƣ���Է�������Խ���»���Խ���۷е�Խ�ߣ�����SiH4�е�ϸߣ��ǽ�����Խǿ�����⻯��Խ�ȶ����ǽ����ԣ�C��Si�����ȶ��Խϸߵ���CH4��
�ʴ�Ϊ��SiH4����ɽṹ�������ʣ���Է�������Խ���»���Խ���۷е�Խ�ߣ�CH4��
��2��SiC��C��Si�γ�4���Ҽ���Ϊsp3�ӻ���SiC��C��Si֮��ͨ�����ۼ���ϣ��ʴ�Ϊ��sp3�����ۼ���
��3���ɱ����ڷ��Ӿ��壬������������ԭ�Ӿ��壬
A��CO2��Cԭ���γ�2���Ҽ���û�йµ��Ӷԣ�Ϊsp�ӻ���SiO2��Si��Oԭ���γ�4���Ҽ�������sp3�ӻ�����������ԭ�ӵ��ӻ���ʽ��ͬ����A��ȷ��
B���ɱ����ڷ��Ӿ��壬�ɷ��ӹ��ɣ�������������ԭ�Ӿ�����ԭ�ӹ��ɣ����Թ��ɾ���������ͬ����B��ȷ��
C���ɱ����ڷ��Ӿ��壬���Ӽ���ڷ��Ӽ���������������������ԭ�Ӿ���ԭ�Ӽ��ɹ��ۼ���ϣ����Ծ�����������������ͬ����C��ȷ��
D��CO2��SiO2�����ڹ��ۻ�����������磬���Ծ��嵼������ͬ����D����
E������ʱ���ɱ������������������۵�ܸߣ����Ծ���ӷ��Բ�����E��ȷ��
�ʴ�Ϊ��D��
��4����֪��H=��Ӧ������ܺ�-����������ܺͣ�
1mol��������2molSi-Si��1molSiO2���4molSi-O��1molO2���1molO=O�����H=2��226+498-4��460=-890kJ?mol-1����Si��s��+O2��g��=SiO2��s����H=-989.2kJ?mol-1��
�ʴ�Ϊ��Si��s��+O2��g��=SiO2��s����H=-989.2kJ?mol-1��
��5��Ԫ�صķǽ�����Խǿ����縺��Խǿ���ʰ���H2N-CH2-COOH�У���Ԫ�صķǽ����ԣ�O��N��C��H����縺�ԣ�O��N��C��H��
�ʴ�Ϊ��O��N��C��H��
�ʴ�Ϊ��SiH4����ɽṹ�������ʣ���Է�������Խ���»���Խ���۷е�Խ�ߣ�CH4��
��2��SiC��C��Si�γ�4���Ҽ���Ϊsp3�ӻ���SiC��C��Si֮��ͨ�����ۼ���ϣ��ʴ�Ϊ��sp3�����ۼ���
��3���ɱ����ڷ��Ӿ��壬������������ԭ�Ӿ��壬
A��CO2��Cԭ���γ�2���Ҽ���û�йµ��Ӷԣ�Ϊsp�ӻ���SiO2��Si��Oԭ���γ�4���Ҽ�������sp3�ӻ�����������ԭ�ӵ��ӻ���ʽ��ͬ����A��ȷ��
B���ɱ����ڷ��Ӿ��壬�ɷ��ӹ��ɣ�������������ԭ�Ӿ�����ԭ�ӹ��ɣ����Թ��ɾ���������ͬ����B��ȷ��
C���ɱ����ڷ��Ӿ��壬���Ӽ���ڷ��Ӽ���������������������ԭ�Ӿ���ԭ�Ӽ��ɹ��ۼ���ϣ����Ծ�����������������ͬ����C��ȷ��
D��CO2��SiO2�����ڹ��ۻ�����������磬���Ծ��嵼������ͬ����D����
E������ʱ���ɱ������������������۵�ܸߣ����Ծ���ӷ��Բ�����E��ȷ��
�ʴ�Ϊ��D��
��4����֪��H=��Ӧ������ܺ�-����������ܺͣ�
1mol��������2molSi-Si��1molSiO2���4molSi-O��1molO2���1molO=O�����H=2��226+498-4��460=-890kJ?mol-1����Si��s��+O2��g��=SiO2��s����H=-989.2kJ?mol-1��
�ʴ�Ϊ��Si��s��+O2��g��=SiO2��s����H=-989.2kJ?mol-1��
��5��Ԫ�صķǽ�����Խǿ����縺��Խǿ���ʰ���H2N-CH2-COOH�У���Ԫ�صķǽ����ԣ�O��N��C��H����縺�ԣ�O��N��C��H��
�ʴ�Ϊ��O��N��C��H��
���������⿼�����⻯��ķе�Ƚϡ��⻯����ȶ��ԡ����塢�ӻ����͡���Ӧ�ȵļ��㡢�縺�Եȣ���Ŀ�漰��֪ʶ��϶࣬�����ڻ���֪ʶ���ۺ�Ӧ�õĿ��飬�Ѷ��еȣ�

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
��Ӧ��A��B����ɫ��Һ������AB�ǰ�ɫ�����������и������·����ķ�Ӧ��A+B�TAB�����ȿ����а�ɫ�������ɵ��ǣ�������
| A�������£�0.1mol?L-1��A��B��Һ��10mL���� |
| B���ڱ�״���£�0.1mol?L-1��A��B��Һ��l0mL���� |
| C���ڱ�״���£�100mL�к�A��B��0.05mol?L-1����Һ |
| D�������£�20mL�к���A��B��0.003mol?L-1����Һ |
ʵ���ã�101kPaʱ��1mol H2��ȫȼ������Һ̬ˮ���ų�285.8kJ��������1mol CH4��ȫȼ������Һ̬ˮ��CO2���ų�890.3kJ�������������Ȼ�ѧ����ʽ����д��ȷ���ǣ�������
��CH4��g��+2O2��g���TCO2��g��+2H2O��l����H=+890.3kJ?mol-1
��CH4��g��+2O2��g���TCO2��g��+2H2O��l����H=-890.3kJ?mol-1
��CH4��g��+2O2��g���TCO2��g��+2H2O��g����H=-890.3kJ?mol-1
��2H2��g��+O2��g���T2H2O��l����H=-571.6kJ?mol-1��
��CH4��g��+2O2��g���TCO2��g��+2H2O��l����H=+890.3kJ?mol-1
��CH4��g��+2O2��g���TCO2��g��+2H2O��l����H=-890.3kJ?mol-1
��CH4��g��+2O2��g���TCO2��g��+2H2O��g����H=-890.3kJ?mol-1
��2H2��g��+O2��g���T2H2O��l����H=-571.6kJ?mol-1��
| A�����Т� | B�����Тڢ� |
| C�����Тڢۢ� | D��ȫ������Ҫ�� |
���з�Ӧ�У����Ĺ̶����õ��ǣ�������
| A��NH3������������NO |
| B��N2��O2��һ�������ºϳ�NO |
| C��NO��O2��Ӧ����NO2 |
| D����NH3��̼����� |

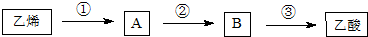
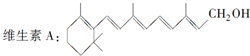 �����л��������̼����Ԫ�ط���ʡ�ԣ���ֻ��ʾ�����м������������ÿ���յ���յ����ʾһ��̼ԭ�ӣ���Ϊ����ʽ�������ļ���ʽΪ�������ά���ص���Ҫ�����٣���һ��ȱ��������Ͳ�����������������������������ά����A�Ľṹ��ʽ��
�����л��������̼����Ԫ�ط���ʡ�ԣ���ֻ��ʾ�����м������������ÿ���յ���յ����ʾһ��̼ԭ�ӣ���Ϊ����ʽ�������ļ���ʽΪ�������ά���ص���Ҫ�����٣���һ��ȱ��������Ͳ�����������������������������ά����A�Ľṹ��ʽ��