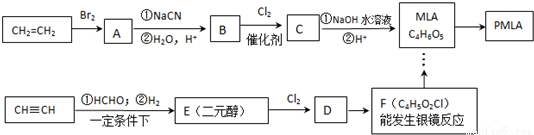
��Ŀ����
����ȫ������Ϲ����к���ƻ���ᣨMLA����ƻ���ᾭ�ۺ����ɾ�ƻ���ᣨPMLA����
��֪��
��1��0.1molƻ����������NaHCO3��Һ��Ӧ�ܲ���4.48L CO2����״������
��2��ƻ������ˮ������ʹ��ˮ��ɫ�IJ��
��3��R-CH2-COOH

RCH2Br
RCH2COOH��

��ش�
��1��д��ʵ��������ϩ�Ļ�ѧ����ʽ
��2��E�ĺ˴Ź�������������壬��������Ϊ1��2��2��E�Ľṹ��ʽΪ
��3��д��MLA���������ŵ�����
��4��д��һ����MLA������ͬ�����ŵ�ͬ���칹��Ľṹ��ʽ
 ��
��
��5������D���Ƿ�����Ԫ�صķ����ǣ�ȡ����D���Թ��У�
��6��д��C��NaOH��Һ��Ӧ�Ļ�ѧ����ʽ
��7��PMLA�������õ����������ԣ�����Ϊ��������ߵȲ���Ӧ��������ҽҩ�������������������������ˮ��Ļ�ѧ����ʽ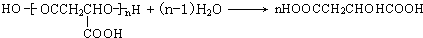
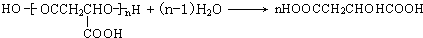 ��
��
��֪��
��1��0.1molƻ����������NaHCO3��Һ��Ӧ�ܲ���4.48L CO2����״������
��2��ƻ������ˮ������ʹ��ˮ��ɫ�IJ��
��3��R-CH2-COOH
| Cl2 |
| ���� |

RCH2Br
| ��NaCN |
| ��H2O��H+ |

��ش�
��1��д��ʵ��������ϩ�Ļ�ѧ����ʽ
CH3CH2OH
H2C=CH2��+H2O
| Ũ���� |
| 170�� |
CH3CH2OH
H2C=CH2��+H2O
��| Ũ���� |
| 170�� |
��2��E�ĺ˴Ź�������������壬��������Ϊ1��2��2��E�Ľṹ��ʽΪ
HOCH2CH2CH2CH2OH
HOCH2CH2CH2CH2OH
����3��д��MLA���������ŵ�����
�Ȼ����ǻ�
�Ȼ����ǻ�
��Fת����MLA���ܷ����ķ�Ӧ����������Ӧ��ȡ����Ӧ
������Ӧ��ȡ����Ӧ
����4��д��һ����MLA������ͬ�����ŵ�ͬ���칹��Ľṹ��ʽ


��5������D���Ƿ�����Ԫ�صķ����ǣ�ȡ����D���Թ��У�
���Թ��м�����������������Һ�����ȣ�ʹ���ַ�Ӧ����ȴ��ȡ����ˮ����Һ�����뵽�������������������Ļ����Һ�У����ְ�ɫ����
���Թ��м�����������������Һ�����ȣ�ʹ���ַ�Ӧ����ȴ��ȡ����ˮ����Һ�����뵽�������������������Ļ����Һ�У����ְ�ɫ����
����֤��D�к�����Ԫ�أ���6��д��C��NaOH��Һ��Ӧ�Ļ�ѧ����ʽ
HOOCCH2CH��Cl��COOH+3NaOH��NaOOCCH2CH��OH��COONa+NaCl+2H2O
HOOCCH2CH��Cl��COOH+3NaOH��NaOOCCH2CH��OH��COONa+NaCl+2H2O
����7��PMLA�������õ����������ԣ�����Ϊ��������ߵȲ���Ӧ��������ҽҩ�������������������������ˮ��Ļ�ѧ����ʽ
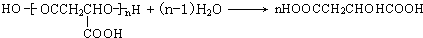
������ƻ�������ʽΪC4H6O5��0��l molƻ����������NaHCO3��Һ��Ӧ�ܲ���4.48L CO2����״������������̼�����ʵ���Ϊ0.2mol����1molƻ���Ậ2mol-COOH��ƻ������ˮ������ʹ��ˮ��ɫ�IJ�����ƻ����ķ���ʽ֪��ƻ����Ľṹ��ʽΪ��HOOCCH2CH��OH��COOH��ƻ����������Ӧ���еľۺ����ɾ�ƻ���ᣨPMLA������ṹΪ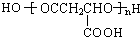 ��
��
��ϩ���巢���ӳɷ�Ӧ����A����AΪBrCH2CH2Br��A������Ϣ��Ӧ���ȷ���ȡ����Ӧ���ٷ���ˮ������B����BΪHOOCH2CH2COOH��B����������ȡ����Ӧ����C�����ƻ����Ľṹ��֪��CΪHOOCCH2CH��Cl��COOH��
��F�ķ���ʽ��֪����Ȳ��HCHO�����������ӳɷ�Ӧ����E��EΪHOCH2CH2CH2CH2OH��F�ܷ�Ӧ������Ӧ������ȩ��-CHO��F����ϵ��ת������ƻ���ᣬ���ƻ����Ľṹ��F�ķ���ʽ����֪FΪOHCCH2CH��Cl��CHO�����C��F�Ľṹ��֪��DΪHOCH2CH2CH��Cl��CH2OH�������л���Ľṹ�����ʽ��
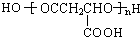 ��
����ϩ���巢���ӳɷ�Ӧ����A����AΪBrCH2CH2Br��A������Ϣ��Ӧ���ȷ���ȡ����Ӧ���ٷ���ˮ������B����BΪHOOCH2CH2COOH��B����������ȡ����Ӧ����C�����ƻ����Ľṹ��֪��CΪHOOCCH2CH��Cl��COOH��
��F�ķ���ʽ��֪����Ȳ��HCHO�����������ӳɷ�Ӧ����E��EΪHOCH2CH2CH2CH2OH��F�ܷ�Ӧ������Ӧ������ȩ��-CHO��F����ϵ��ת������ƻ���ᣬ���ƻ����Ľṹ��F�ķ���ʽ����֪FΪOHCCH2CH��Cl��CHO�����C��F�Ľṹ��֪��DΪHOCH2CH2CH��Cl��CH2OH�������л���Ľṹ�����ʽ��
����⣺ƻ�������ʽΪC4H6O5��0��l molƻ����������NaHCO3��Һ��Ӧ�ܲ���4.48L CO2����״������������̼�����ʵ���Ϊ0.2mol����1molƻ���Ậ2mol-COOH��ƻ������ˮ������ʹ��ˮ��ɫ�IJ�����ƻ����ķ���ʽ֪��ƻ����Ľṹ��ʽΪ��HOOCCH2CH��OH��COOH��ƻ����������Ӧ���еľۺ����ɾ�ƻ���ᣨPMLA������ṹΪ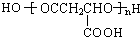 ��
��
��ϩ���巢���ӳɷ�Ӧ����A����AΪBrCH2CH2Br��A������Ϣ��Ӧ���ȷ���ȡ����Ӧ���ٷ���ˮ������B����BΪHOOCH2CH2COOH��B����������ȡ����Ӧ����C�����ƻ����Ľṹ��֪��CΪHOOCCH2CH��Cl��COOH��
��F�ķ���ʽ��֪����Ȳ��HCHO�����������ӳɷ�Ӧ����E��EΪHOCH2CH2CH2CH2OH��F�ܷ�Ӧ������Ӧ������ȩ��-CHO��F����ϵ��ת������ƻ���ᣬ���ƻ����Ľṹ��F�ķ���ʽ����֪FΪOHCCH2CH��Cl��CHO�����C��F�Ľṹ��֪��DΪHOCH2CH2CH��Cl��CH2OH��
��1��ʵ��������ϩ�Ļ�ѧ����ʽΪ��CH3CH2OH
H2C=CH2��+H2O��
�ʴ�Ϊ��CH3CH2OH
H2C=CH2��+H2O��
��2��������������֪��E�Ľṹ��ʽΪHOCH2CH2CH2CH2OH��
�ʴ�Ϊ��HOCH2CH2CH2CH2OH��
��3��������������֪��MLAΪHOOCCH2CH��OH��COOH�������Ȼ����ǻ���
FΪOHCCH2CH��Cl��CHO��Fת����MLA���ܷ����ķ�Ӧ����Ϊ������Ӧ��ȡ����Ӧ��
�ʴ�Ϊ���Ȼ����ǻ���������Ӧ��ȡ����Ӧ��
��4����MLA������ͬ�����ŵ�ͬ���칹��Ľṹ��ʽΪ�� ��
��
�ʴ�Ϊ�� ��
��
��5��DΪHOCH2CH2CH��Cl��CH2OH������D���Ƿ�����Ԫ�صķ����ǣ�ȡ����D���Թ��У����Թ��м�����������������Һ�����ȣ�ʹ���ַ�Ӧ����ȴ��ȡ����ˮ����Һ�����뵽�������������������Ļ����Һ�У����ְ�ɫ��������֤��D�к�����Ԫ�أ�
�ʴ�Ϊ�����Թ��м�����������������Һ�����ȣ�ʹ���ַ�Ӧ����ȴ��ȡ����ˮ����Һ�����뵽�������������������Ļ����Һ�У����ְ�ɫ������
��6��CΪHOOCCH2CH��Cl��COOH����NaOH��Һ��Ӧ�Ļ�ѧ����ʽΪ��HOOCCH2CH��Cl��COOH+3NaOH��NaOOCCH2CH��OH��COONa+NaCl+2H2O��
�ʴ�Ϊ��HOOCCH2CH��Cl��COOH+3NaOH��NaOOCCH2CH��OH��COONa+NaCl+2H2O��
��7��PMLA�������õ����������ԣ�����Ϊ��������ߵȲ���Ӧ��������ҽҩ�������������������������ˮ��Ļ�ѧ����ʽΪ��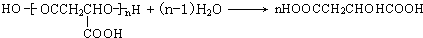 ��
��
�ʴ�Ϊ��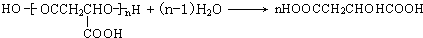 ��
��
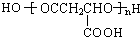 ��
����ϩ���巢���ӳɷ�Ӧ����A����AΪBrCH2CH2Br��A������Ϣ��Ӧ���ȷ���ȡ����Ӧ���ٷ���ˮ������B����BΪHOOCH2CH2COOH��B����������ȡ����Ӧ����C�����ƻ����Ľṹ��֪��CΪHOOCCH2CH��Cl��COOH��
��F�ķ���ʽ��֪����Ȳ��HCHO�����������ӳɷ�Ӧ����E��EΪHOCH2CH2CH2CH2OH��F�ܷ�Ӧ������Ӧ������ȩ��-CHO��F����ϵ��ת������ƻ���ᣬ���ƻ����Ľṹ��F�ķ���ʽ����֪FΪOHCCH2CH��Cl��CHO�����C��F�Ľṹ��֪��DΪHOCH2CH2CH��Cl��CH2OH��
��1��ʵ��������ϩ�Ļ�ѧ����ʽΪ��CH3CH2OH
| Ũ���� |
| 170�� |
�ʴ�Ϊ��CH3CH2OH
| Ũ���� |
| 170�� |
��2��������������֪��E�Ľṹ��ʽΪHOCH2CH2CH2CH2OH��
�ʴ�Ϊ��HOCH2CH2CH2CH2OH��
��3��������������֪��MLAΪHOOCCH2CH��OH��COOH�������Ȼ����ǻ���
FΪOHCCH2CH��Cl��CHO��Fת����MLA���ܷ����ķ�Ӧ����Ϊ������Ӧ��ȡ����Ӧ��
�ʴ�Ϊ���Ȼ����ǻ���������Ӧ��ȡ����Ӧ��
��4����MLA������ͬ�����ŵ�ͬ���칹��Ľṹ��ʽΪ��
 ��
���ʴ�Ϊ��
 ��
����5��DΪHOCH2CH2CH��Cl��CH2OH������D���Ƿ�����Ԫ�صķ����ǣ�ȡ����D���Թ��У����Թ��м�����������������Һ�����ȣ�ʹ���ַ�Ӧ����ȴ��ȡ����ˮ����Һ�����뵽�������������������Ļ����Һ�У����ְ�ɫ��������֤��D�к�����Ԫ�أ�
�ʴ�Ϊ�����Թ��м�����������������Һ�����ȣ�ʹ���ַ�Ӧ����ȴ��ȡ����ˮ����Һ�����뵽�������������������Ļ����Һ�У����ְ�ɫ������
��6��CΪHOOCCH2CH��Cl��COOH����NaOH��Һ��Ӧ�Ļ�ѧ����ʽΪ��HOOCCH2CH��Cl��COOH+3NaOH��NaOOCCH2CH��OH��COONa+NaCl+2H2O��
�ʴ�Ϊ��HOOCCH2CH��Cl��COOH+3NaOH��NaOOCCH2CH��OH��COONa+NaCl+2H2O��
��7��PMLA�������õ����������ԣ�����Ϊ��������ߵȲ���Ӧ��������ҽҩ�������������������������ˮ��Ļ�ѧ����ʽΪ��
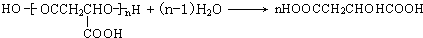 ��
���ʴ�Ϊ��
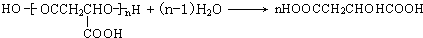 ��
�����������⿼���л�����ƶ���ϳɡ��Ը�����Ϣ�����á������ŵ�������ת������ȷƻ������������ƶ���ṹ�ǽ����Ĺؼ���ע����ݷ�Ӧ��Ϣ�����ƶϣ���Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
�����Ŀ

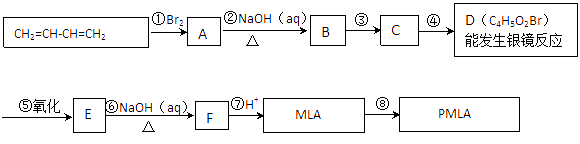


 RCH2COOH��
RCH2COOH��