��Ŀ����
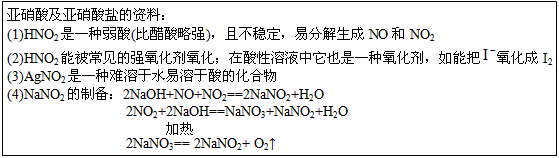
��1������������Ѫ�쵰����Ӧ��Fe2+������ʳ�������Σ���NaNO2��������Ѫ�쵰����Fe2+ת��Ϊ
Fe3+���ж�������ά����C�ɽⶾ��������������ȷ����________��
A�����������ǻ�ԭ�� B��ά����C�ǻ�ԭ�� C��ά����C��Fe3+��ԭΪFe2+ D���������α���ԭ
��2���������������������NaNO2��NaCl����________��
A���ⶨ��������Һ��pH
B����AgNO3��HNO3�����Լ�������
C�������������¼���KI������Һ������
D���ֱ�����������Һ�еμӼ���
��3��Ϊ�˲ⶨij��Ʒ��NaNO2�ĺ���������ʹ�ñ�KMnO4��Һ���еζ����Իش�
��KMnO4��Һ�ڵζ���������________����������� ����ԭ���������õζ�����________���Ҫ����Ҫ��������ָʾ����
�����ζ��յ����ʱĿ�⸩�ӣ������ý��________���ƫ��ƫС������Ӱ�족����
��4��ijͬѧ�ڼ��������м���NaCl��NaNO2��ʵ�飬��������ֻ��Ũ���ᣬ���ʸ�ʵ���ܷ�ɹ���˵�����ɡ�_______________________________________
��5�����е������ֳ�����������ɵĻ������44.8L������ѻ���ɱ�״̬��������N2O4��NO���������Ϊ20%��
�ٽ�������ͨ��������NaOH��Һ��ǡ�÷�Ӧ������Һ��NaNO2��������__________g��
�ڷ�Ӧ�����Һ����������NaNO2���ʵ���__________mol��
��2��D
��3��������������Ҫ����ƫС
��4���ܣ�ǿ����ȡ���ᣬ���ɵ�HNO2�ֽ�Ϊ����ɫNO2����
��5����110.4����2.4

| |||||||||||||||||||||||||||||||||||||||
