��Ŀ����
˫��ˮ��H2O2����ˮ���Ǽ����ĵ���ʣ���H2O2��H2O�������ԡ�
��1������H2O2�����Ƕ�Ԫ���ᣬ��д������ˮ�еĵ��뷽��ʽ��
��2������H2O2�������ԣ�����ͬǿ�������γ����Σ���һ��������Ҳ���γ���ʽ�Ρ���д��H2O2��Ba(OH)2�����γ����εĻ�ѧ����ʽ�� ��
��3��ˮ��������H3O����OH������ˮ����ż���롣ͬˮһ����H2O2Ҳ�м�������ż���룬����ż����ķ���ʽ��
��1��H2O2 H��+HO2- HO2-
H��+HO2- HO2-  2H��+O22��
2H��+O22��
��2��H2O2+Ba(OH)2=BaO2+2H2O
��3��H2O2+H2O2 H3O2��+HO2-
H3O2��+HO2-
���������������1������������������ʣ�H2O2��Һ���������ڶ�Ԫ����ֲ����룬����ķ���ʽΪ�� H2O2?H++HO2- HO2-?H++O22-����2��H2O2��������,����Ba(OH)2��Ӧ�����ɿ���Ϊ����кͷ�Ӧ���䷴Ӧ����ʽΪ��H2O2+Ba(OH)2=BaO2+2H2O��3����ˮ��������Ӧ����ʽ��H2O+H2O H3O��OH����֪��ˮ����������ӣ��γ�ˮ�������ӡ���H2O2���뷽��ʽΪ��H2O2+H2O2
H3O��OH����֪��ˮ����������ӣ��γ�ˮ�������ӡ���H2O2���뷽��ʽΪ��H2O2+H2O2 H3O2��+HO2-
H3O2��+HO2-
���㣺���뷽��ʽ����д�����ӷ���ʽ����д

��������10 mL 0.1mol��L�C1��ˮ�л���������ˮϡ�͵�1 L�����б仯����ȷ����
�ٵ���̶�����c(H+)���۵�������ǿ���� ���䣻��OH�C��Ŀ����H+��Ŀ��С����pH���� c(H+)��c(OH�C)�ij˻���С
���䣻��OH�C��Ŀ����H+��Ŀ��С����pH���� c(H+)��c(OH�C)�ij˻���С
| A���٢ڢ� | B���٢ݢ� | C�������ⶼ��ȷ | D���٢ܢݢޢ� |
�����£��� 0��1000 mol��L-1NaOH��Һ�ζ�20��00 mL 0��1000 mol��L-1CH3COOH��Һ���õζ�������ͼ������˵����ȷ����
| A�������ʾ��Һ�У�c(CH3COO��) +2 c(OH��)=c(CH3COOH)+2c(H+) |
| B�������ʾ��Һ�У�c(Na+)��c(CH3COO��)+c(CH3COOH) |
| C�������ʾ��Һ�У�c(Na+)��c(OH��)��c(CH3COO��)��c(H+) |
| D���ζ������п��ܳ��֣�c(CH3COOH)��c(H+)��c(CH3COO��)��c(Na+)��c(OH��) |
��20mL0��1mol/L �Ĵ�����Һ�У���ʹ��Һ��pH��С������ʹ����ĵ���ƽ�����淽���ƶ����ɼ�����Լ���
| A��20mLˮ | B��Ũ���� | C�������� | D��NaOH��Һ |
AgCl��s��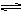 Ag+��aq��+Cl-��aq����ƽ��ʱ��Ksp=C��Ag+ )��C(Cl-) ,�����Ȼ����ֱ�Ͷ���100 mLˮ ��24 mL 0.1 mol��L-1NaCl ��10 mL 0.1 mol��L-1MgCl2 ��30 mL 0.1 mol��L-1AgNO3��Һ��,��Һ��C(Ag+)�ɴ�С˳��Ϊ
Ag+��aq��+Cl-��aq����ƽ��ʱ��Ksp=C��Ag+ )��C(Cl-) ,�����Ȼ����ֱ�Ͷ���100 mLˮ ��24 mL 0.1 mol��L-1NaCl ��10 mL 0.1 mol��L-1MgCl2 ��30 mL 0.1 mol��L-1AgNO3��Һ��,��Һ��C(Ag+)�ɴ�С˳��Ϊ
| A���ۢ٢ڢ� | B���ܢ٢ڢ� | C���٢ڢۢ� | D���ܢۢڢ� |
0.1mol��L��1Na2S��Һ�����й�ϵ��ȷ����
| A��c(Na+)=2c(S2��) |
| B��c(OH��)="c" (H+)+c (HS��)+2c (H2S) |
| C��c(Na+) ��c(S2��) ��c (HS��) ��c (OH��) |
| D��c(Na+)+c (H+)="2c" (S2��)+c (HS��)+c (OH��) |
25��ʱ��ˮ�ĵ���ﵽƽ�⣺H2O H����OH������H>0������������ȷ����
H����OH������H>0������������ȷ����
| A����ˮ�м���ϡ��ˮ��ƽ�������ƶ���c(OH��)���� |
| B����ˮ�м������������������ƣ�c(H��)����KW���� |
| C����ˮ��ͨ��HCl���壬ƽ�������ƶ�����Һ������������ |
| D����ˮ���ȣ�KW����pH���� |
����˵���У���ȷ����
| A��ij���ӱ�������ȫ��ָ����������Һ�е�Ũ�ȱ�Ϊ0 |
| B��ij���ʵ��ܽ���Ϊ���ܣ�������ʵ��ܽ��Ϊ0 |
| C��һ����˵���ܽ��С������������ת��Ϊ�ܽ�ȴ�������� |
| D�����������ɡ��ܽ��ת����ʵ�ʶ��dz����������ܽ�ƽ����ƶ� |