��Ŀ����
(14��A��B��C�� D ��E��F�dz��������壬����A��B��C��DΪ���ʣ��йص�ת����ϵ����ͼ��ʾ(��Ӧ����������ȥ����
D ��E��F�dz��������壬����A��B��C��DΪ���ʣ��йص�ת����ϵ����ͼ��ʾ(��Ӧ����������ȥ����
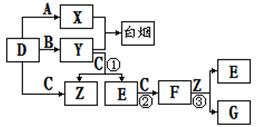
��ش��������⣺
(1)D�ĵ���ʽΪ ��
(2)��Ӧ �۵����ӷ���ʽΪ ��
�۵����ӷ���ʽΪ ��
(3)Y��E��һ�������¿ɷ�Ӧ����B��Z��������E�Ի�������Ⱦ���÷�Ӧ�Ļ�ѧ����ʽ Ϊ ��
Ϊ ��
(4)������0.1mol/L��Y��Һ��c(H+)/c(OH-)=1��10-8������������ȷ���� ��
�ٸ���Һ��pH=11
�ڸ���Һ�е����ʵ������������Ũ��Ϊ0.1mol/L
�۽�pH=11��Y��Һ��ˮϡ��100����pHֵΪ9
�ܸ� ��Һ��ˮ�������c(H+)��c(OH-)�˻�Ϊ1��10-22
��Һ��ˮ�������c(H+)��c(OH-)�˻�Ϊ1��10-22
��0.1mol/L��������ҺV1 L���0.1mol/L��Y��ҺV2 L��ϣ��������ҺpH=7����:V1>V2
(5)������pH=a��X��Һ��pH=b��Y��Һ�������ϣ���a+b=14�����Ϻ����Һ��________�ԣ������Һ�и�����Ũ�ȴ�С��ϵΪ______ ______________��
 D ��E��F�dz��������壬����A��B��C��DΪ���ʣ��йص�ת����ϵ����ͼ��ʾ(��Ӧ����������ȥ����
D ��E��F�dz��������壬����A��B��C��DΪ���ʣ��йص�ת����ϵ����ͼ��ʾ(��Ӧ����������ȥ����
��ش��������⣺
(1)D�ĵ���ʽΪ ��
(2)��Ӧ
 �۵����ӷ���ʽΪ ��
�۵����ӷ���ʽΪ ��(3)Y��E��һ�������¿ɷ�Ӧ����B��Z��������E�Ի�������Ⱦ���÷�Ӧ�Ļ�ѧ����ʽ
 Ϊ ��
Ϊ ��(4)������0.1mol/L��Y��Һ��c(H+)/c(OH-)=1��10-8������������ȷ���� ��
�ٸ���Һ��pH=11
�ڸ���Һ�е����ʵ������������Ũ��Ϊ0.1mol/L
�۽�pH=11��Y��Һ��ˮϡ��100����pHֵΪ9
�ܸ�
 ��Һ��ˮ�������c(H+)��c(OH-)�˻�Ϊ1��10-22
��Һ��ˮ�������c(H+)��c(OH-)�˻�Ϊ1��10-22��0.1mol/L��������ҺV1 L���0.1mol/L��Y��ҺV2 L��ϣ��������ҺpH=7����:V1>V2
(5)������pH=a��X��Һ��pH=b��Y��Һ�������ϣ���a+b=14�����Ϻ����Һ��________�ԣ������Һ�и�����Ũ�ȴ�С��ϵΪ______ ______________��
(14��)(1)H�sH ��2�֣� (2)3NO2+H2O=2H++2NO3-+NO ��3�֣�
(3)4NH3+6NO
(4)�٢� ��2�֣� (5)�� ��2�֣� c(NH4+)> c(Cl-)>c(OH-)>c(H+)��2�֣�
(3)4NH3+6NO

(4)�٢� ��2�֣� (5)�� ��2�֣� c(NH4+)> c(Cl-)>c(OH-)>c(H+)��2�֣�
��

��ϰ��ϵ�д�
 ��ʦָ����ĩ��̾�ϵ�д�
��ʦָ����ĩ��̾�ϵ�д�
�����Ŀ
 �����������ж���ȷ���ǣ� ��
�����������ж���ȷ���ǣ� �� ����ˮ��Һ��ַ�Ӧ����������ǿ�ᣬ�÷�Ӧ�Ļ�ѧ����ʽ
����ˮ��Һ��ַ�Ӧ����������ǿ�ᣬ�÷�Ӧ�Ļ�ѧ����ʽ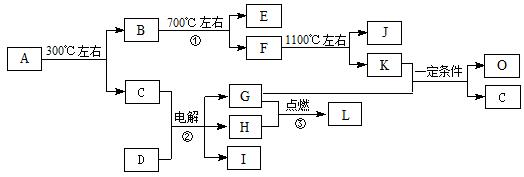
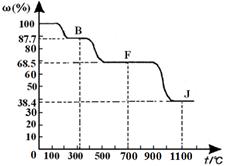

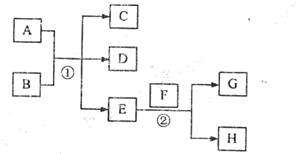
 ��C��D֮�䣬B��C��D�о����м�Ԫ�أ�д������ʱ�ٵ����ӷ���ʽ�� ��
��C��D֮�䣬B��C��D�о����м�Ԫ�أ�д������ʱ�ٵ����ӷ���ʽ�� �� B+H�ܷų��������ȣ��÷�Ӧ��Ӧ��������ĺ��ӣ�
B+H�ܷų��������ȣ��÷�Ӧ��Ӧ��������ĺ��ӣ� 2F+D��F��EԪ�ص���������Ϊ60%��
2F+D��F��EԪ�ص���������Ϊ60%��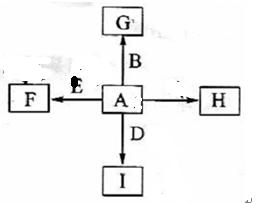
 ��
��
 C��E�Ļ�ѧ����ʽΪ
C��E�Ļ�ѧ����ʽΪ  ��D��Ԫ������������Ӧˮ������Ե�ǿ�����ѧʽ�� ��
��D��Ԫ������������Ӧˮ������Ե�ǿ�����ѧʽ�� �� ________________________________��
________________________________��