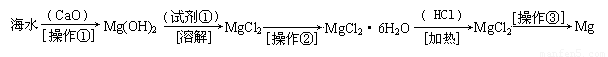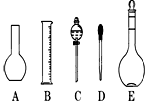��Ŀ����
��1��MgO ��Al2O3��
��3��FeCl3��FeCl2��
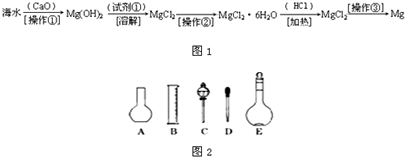
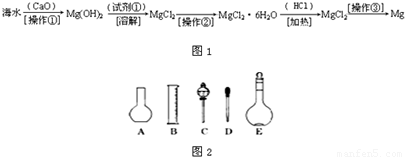
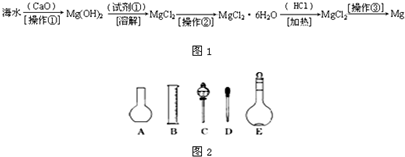
��������ˮ�к��д������Ȼ�þ���Ӻ�ˮ����ȡþ������������ͼ1��ʾ��
�ش��������⣺
д���ں�ˮ�м�������������������þ�Ļ�ѧ����ʽ
��������Ҫ��ָ
������ʵ��������480ml 0.1mol?L-1��Na2CO3��Һ���ش��������⣺
��1��Ӧ��������ƽ��ȡʮˮ̼���ƾ���
��2����ͼ2��ʾ������������Һ�϶�����Ҫ����
��3������ƿ�ϱ��У����¶ȡ���Ũ�ȡ�����������ѹǿ���ݿ̶��ߡ�����ʽ���ʽ�������е�
��4�������������Ҫ�����ǣ�a����ƿ��b�ձ���c��ͷ�ιܡ�d������ƽ�������ڲ���������ʹ�õ�ǰ��˳����
��5���������ǻ�ѧʵ���г��õ�һ�ֲ������ߣ�����������Һ�Ĺ����в�����������
��6����ʵ��ʱ���������������ʹ��Һ��Ũ��ƫ�͵���
A������ǰ���н�����ƿ�е�ˮ������ B��̼����ʧȥ�˲��ֽᾧˮ��
C��̼���ƾ��岻�������л����Ȼ��ƣ� D������̼���ƾ���ʱ�����������⣻ E������ʱ���ӿ̶��ߣ�
��2��Cl2��HCl���Ȼ���������ʳ��ˮ�����������ܣ�
��3��FeCl3��FeCl2�� ��ȥFeCl3��Һ�е�����FeCl2��ѡ����������ˮ�ȣ�
��4��NaHCO3��Һ��Na2CO3�� Na2CO3��Һ���������̼��Ӧ����NaHCO3��
��������ˮ�к��д������Ȼ�þ���Ӻ�ˮ����ȡþ��������������ˮ��Ӧ�õ��������ƣ����������뺣ˮ���Ȼ�þ��Ӧ����������þ�������Ȼ��ƣ����˳�����������þ��ϡ���ᷴӦ�������Ȼ�þ��ˮ������Ȼ�þ���ɽ���þ���������ݴ˽�ϸ�С�ʼ��ɽ��
��������1������n=cv����̼���Ƶ����ʵ���������Na2CO3?10H2O�����ʵ�������Na2CO3�����ʵ���������m=nM����Na2CO3?10H2O��������
��2������ʵ������IJ����Լ�ÿ��������Ҫ����ȷ����Ӧ��������������û��480mL������ƿ��Ӧѡ�����480mL�ҹ�����������ƿ��
��3����������ƿ�ϱ����¶ȡ��������̶��߽��н��
��4������������Һ��ʵ��������̽�������ʹ������
��5���ܽ��Dz��������ڽ�������ܽ⣬��Һʱ������
��6���������������ʵ����ʵ��������Һ�������Ӱ�죬����C=
| n |
| V |
��2��Cl2��HCl���Ȼ��⼫������ˮ������������ˮ������ˮ��Ӧ��Cl2+H2O=H++Cl-+HClO��ʳ��ˮ��Һ�е������������������ܽ⣬�����������ܽ�ȣ����Կ��ñ���ʳ��ˮ��ȥ�����л��е��Ȼ������壬�ʴ�Ϊ������NaCl��Һ��
��3��FeCl3��FeCl2�� ��ȥFeCl3��Һ�е�����FeCl2��ѡ����������ˮ�ȣ����������ӷ�ӦΪ2Fe2++Cl2=2Fe3++2Cl-���ʴ�Ϊ����������ˮ��
��4��NaHCO3��Һ��Na2CO3�� ��ȥNaHCO3��Һ�к��е�Na2CO3���ʿ�����Һ��ͨ�����������̼���壬Na2CO3��Һ���������̼��Ӧ����NaHCO3����Ӧ�����ӷ���ʽΪCO32-+H2O+CO2=2HCO3-���ʴ�Ϊ��CO2��
�������ں�ˮ�м��������ƣ������ƺ�ˮ��Ӧ�������������ƣ�CaO+H2O=Ca��OH��2�٣��������ƺ��Ȼ�þ��Ӧ������������þ�������Ȼ��ƣ�Ca��OH��2+MgCl2=Mg��OH��2��+CaCl2�ڣ��ܵķ���ʽ��+�ڵã�CaO+MgCl2+H2O=Mg��OH��2+CaCl2�������ƺ�ˮ�������������ƣ��������ƺ��Ȼ�þ������������þ���������Բ���1�������ǹ��ˣ�������þ���������ᷴӦ�����Ȼ�þ��ˮ��Mg��OH��2+2HCl=MgCl2+2H2O�������Լ��ٿ�ѡ�����ᣬ���Ȼ�þ�ܽ�����¶ȵ�Ӱ�����ͨ��ʲô����ʹ���ʴ����ı�����Һ�нᾧ�������Ȼ�þ�ܽ�����¶ȵ�Ӱ��仯�������Բ����ڵ�����������Ũ������ȴ�ᾧ�����ˣ�����Ȼ�þ���ɽ���þ���������÷�Ӧ�Ļ�ѧ����ʽ��MgCl2
| ||
�ʴ�Ϊ��CaO+MgCl2+H2O=Mg��OH��2+CaCl2�����ˣ�HCl������Ũ������ȴ�ᾧ�����ˣ�
��������1��û��480mL������ƿ��Ӧѡ�����480mL�ҹ�����������ƿ����ѡ��500mL����ƿ��
����500mL0.1mol?L-1��Na2CO3��Һ��Ҫ̼���Ƶ�����Ϊ��0.1mol?L-1��0.5L=0.05mol��Na2CO3?10H2O�����ʵ�������Na2CO3�����ʵ���������Na2CO3?10H2O������0.05mol��286g/mol=14.3g��
�ʴ�Ϊ��14.3��
��2������˳���ǣ�������������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȡ�װƿ��ǩ��һ������ƽ�������õ�ҩ�ף����������ձ����ܽ⣬��ȴ��ת�Ƶ�500mL����ƿ�У����ò�����������ת����ϣ�����������ˮϴ���ձ���������2��3�β���ϴ��Һȫ��ת�Ƶ�����ƿ�У��ټ���������ˮ������ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμӣ�ʹ��Һ�İ�Һ�����͵��������ƽ������ƿ�����������µߵ�ҡ�ȣ�������Ҫ������Ϊ��������ƽ��ҩ�ס��ձ�����������500mL����ƿ����ͷ�ιܣ�����ҪԲ����ƿ����Һ©����
�ʴ�Ϊ��AC��500ml��
��3����������ƿ�ϱ����¶ȡ��������̶��ߣ���ѡ���٢ۢݣ�
��4�����������м��㡢�������ܽ⡢��Һ��ϴ����Һ�����ݡ�ҡ�ȵȲ�����һ����������ƽ��������ҩ��ȡ��ҩƷ�����ձ����ܽ⣨������Ͳ��ȡˮ�����ձ��������ò��������裬�����ܽ⣬��ȴ��ת�Ƶ�500mL����ƿ�У����ò�����������ϴ���ձ���������2-3�Σ�����ϴ��Һ��������ƿ�У���ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμӣ�����ݵߵ�ҡ�ȣ����ڲ���������ʹ�õ�ǰ��˳���ǣ�d b a c��
�ʴ�Ϊ��d b a c��
��5���ܽ��Dz��������ڽ�������ܽ⣬��Һʱ�������ã��ʴ�Ϊ��2��
��6��A����Һ�������ˮ���ݣ�����ǰû�н�����ƿ�е�ˮ����������ƿδ�������ʹ�ò�Ӱ�����ʵ����ʵ�����Ҳ��Ӱ����Һ����������Զ����Ƶ���ҺŨ����Ӱ�죬��A����
B��̼����ʧȥ�˲��ֽᾧˮ�����³������ʵ����������������ʵ����ʵ���ƫ��������Һ��Ũ��ƫ�ߣ���B����
C��̼���ƾ��岻�������л����Ȼ��ƣ�����̼���Ƶ����ʵ���ƫС��������Һ��Ũ��ƫ�ͣ���C��ȷ��
D������̼���ƾ���ʱ�����������⣬���³������ʵ����������������ʵ����ʵ���ƫ��������Һ��Ũ��ƫ�ߣ���D����
E��������ʱ���ӿ̶��ߣ�������Һ�����ƫ������������Һ��Ũ��ƫ�ͣ���E��ȷ��
��ѡCE��

 ��У����ϵ�д�
��У����ϵ�д���һ�������е����������ʣ�д����ȥ��Щ���ʵ��Լ���
��1��MgO (Al2O3) ��2��Cl2(HCl) �� ��
��3��FeCl3(FeCl2) �� ��4��NaHCO3��Һ(Na2CO3) ��
������(6��)��ˮ�к��д������Ȼ�þ���Ӻ�ˮ����ȡþ��������������ͼ��ʾ��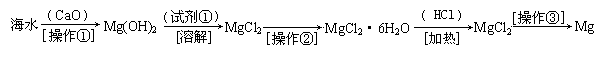
�ش��������⣺
д���ں�ˮ�м�������������������þ�Ļ�ѧ����ʽ���������� ����������������
��������Ҫ��ָ �� �� ���Լ��ٿ�ѡ�� �� �� ��
��������ָ�������� �������� �������������տɵý���þ��
��������8�֣�ʵ��������480ml 0.1mol��L-1��Na2CO3��Һ���ش��������⣺
��1��Ӧ��������ƽ��ȡʮˮ̼���ƾ��� g��
��2����ͼ��ʾ������������Һ�϶�����Ҫ������ �� (�����)����ʵ�����貣������E���Ϊ���� ����mL��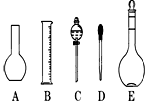
��3������ƿ�ϱ��У����¶ȡ���Ũ�ȡ�����������ѹǿ���ݿ̶��ߡ�����ʽ���ʽ�������е� ���������ַ��ţ�
��4�������������Ҫ�����ǣ�a����ƿ��b�ձ���c��ͷ�ιܡ�d������ƽ�������ڲ���������ʹ�õ�ǰ��˳���� ������д��ĸ��ÿ������ֻ��ѡ��һ�Σ�
��5���������ǻ�ѧʵ���г��õ�һ�ֲ������ߣ�����������Һ�Ĺ����в�����������
����;������д���֣�
��6����ʵ��ʱ���������������ʹ��Һ��Ũ��ƫ�͵��� ��
| A������ǰû�н�����ƿ�е�ˮ������ | B��̼����ʧȥ�˲��ֽᾧˮ�� |
| C��̼���ƾ��岻�������л����Ȼ��ƣ� | D������̼���ƾ���ʱ�����������⣻ |