��Ŀ����
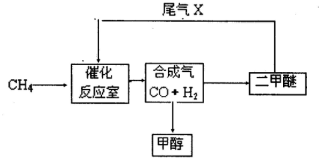 ��2010?�Ϸ���ģ���ϳ�����CO+H2����һ����Ҫ�Ļ���ԭ�ϣ��ڻ��������о���ʮ�ֹ㷺����;�� ������������Ϊ21���͵�����ȼ��--�״��������ѣ�CH3OCH3�������ʣ��乤���������£�
��2010?�Ϸ���ģ���ϳ�����CO+H2����һ����Ҫ�Ļ���ԭ�ϣ��ڻ��������о���ʮ�ֹ㷺����;�� ������������Ϊ21���͵�����ȼ��--�״��������ѣ�CH3OCH3�������ʣ��乤���������£���1��д���úϳ������������ѵĻ�ѧ����ʽ
2CO+4H2
CH3OCH3+H2O
| ||
2CO+4H2
CH3OCH3+H2O
��
| ||
��2��β��ѭ�����漰�����·�Ӧ��CH4��g��+H2O��g��?CO��g��+3H2��g������д��ij�¶��¸÷�Ӧ��ƽ�ⳣ������ʽ
K=
| c(CO)?c3(H2) |
| c(H2O)?c(CH4) |
K=
��| c(CO)?c3(H2) |
| c(H2O)?c(CH4) |
��3����ҵ��һ������������ַ�Ӧ�ϳɼ״���
��Ӧ��CO��g��+2H2��g��?CH3OH��g����H1
��Ӧ��CO2��g��+3H2��g��?CH3��g��+H2O��g����H2
���±����������Ƿ�Ӧ�ڲ�ͬ�¶��µĻ�ѧƽ�ⳣ����K����
| �¶� | 250 | 300 | 350 |
| K | 2.041 | 0.270 | 0.012 |
��
��
0�����������=�����������������Ѻͣ�CH3OH��g��+
| 1 |
| 2 |
H2��g��+
| 1 |
| 2 |
���H2=
-48.9kJ?mol-1
-48.9kJ?mol-1
����4���Զ����ѡ�����������������ҺΪԭ�ϣ���Ϊ�缫����ȼ�ϵ�أ�д���õ�ظ����ĵ缫��Ӧʽ
CH3OCH3+160H--12e-�T2CO32-+11H2O
CH3OCH3+160H--12e-�T2CO32-+11H2O
����������1������ѭ������ͼ����֪����һ����̼��������һ���������ºϳɼ״���
��2����Ӧ��ƽ�ⳣ������ʽ=
���ش�
��3���ٶ��ڷ��ȷ�Ӧ���¶�Խ�ߣ���ѧƽ�ⳣ��ԽС��
�ڸ��ݸ�˹���������㻯ѧ��Ӧ���ʱ䣻
��4��ȼ�ϵ���У���������ȼ�Ϸ���ʧ���ӵ�������Ӧ��
��2����Ӧ��ƽ�ⳣ������ʽ=
| ����������ƽ��Ũ��ϵ���η��ij˻� |
| ������Ӧ��ƽ��Ũ��ϵ���η��ij˻� |
��3���ٶ��ڷ��ȷ�Ӧ���¶�Խ�ߣ���ѧƽ�ⳣ��ԽС��
�ڸ��ݸ�˹���������㻯ѧ��Ӧ���ʱ䣻
��4��ȼ�ϵ���У���������ȼ�Ϸ���ʧ���ӵ�������Ӧ��
����⣺��1��һ����̼��������һ���������ºϳɼ״��Ļ�ѧ����ʽΪ��2CO+4H2
CH3OCH3+H2O���ʴ�Ϊ��2CO+4H2
CH3OCH3+H2O��
��2����Ӧ��ƽ�ⳣ������ʽ=
��������ʽ��K=
���ʴ�Ϊ��K=
��
��3���ٸ��ݱ������ݿ��Կ������¶�Խ�ߣ���ѧƽ�ⳣ��ԽС�������ڷ��ȷ�Ӧ���ý��۳������ʴ�Ϊ������
�ڸ��ݸ�˹���������㻯ѧ��Ӧ���ʱ�ó���H2=-48.9kJ?mol-1���ʴ�Ϊ��-48.9kJ?mol-1��
��4��ȼ�ϵ���У���������ȼ�ϼ��ѷ���ʧ���ӵ�������Ӧ���ڼ��Ի����£���Ϊ��CH3OCH3+160H--12e-�T2CO32-+11H2O���ʴ�Ϊ��CH3OCH3+160H--12e-�T2CO32-+11H2O��
| ||
| ||
��2����Ӧ��ƽ�ⳣ������ʽ=
| ����������ƽ��Ũ��ϵ���η��ij˻� |
| ������Ӧ��ƽ��Ũ��ϵ���η��ij˻� |
| c(CO)?c3(H2) |
| c(H2O)?c(CH4) |
| c(CO)?c3(H2) |
| c(H2O)?c(CH4) |
��3���ٸ��ݱ������ݿ��Կ������¶�Խ�ߣ���ѧƽ�ⳣ��ԽС�������ڷ��ȷ�Ӧ���ý��۳������ʴ�Ϊ������
�ڸ��ݸ�˹���������㻯ѧ��Ӧ���ʱ�ó���H2=-48.9kJ?mol-1���ʴ�Ϊ��-48.9kJ?mol-1��
��4��ȼ�ϵ���У���������ȼ�ϼ��ѷ���ʧ���ӵ�������Ӧ���ڼ��Ի����£���Ϊ��CH3OCH3+160H--12e-�T2CO32-+11H2O���ʴ�Ϊ��CH3OCH3+160H--12e-�T2CO32-+11H2O��
���������⿼��ѧ���йػ�ѧƽ���Լ��Ȼ�ѧ֪ʶ�����Ը�����ѧ֪ʶ���ش��ѶȽϴ�

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
��2010?�Ϸ���ģ����ͼ��ʾ���������������ʵ�飮ʵ��ʱ��NaOH�����ϵμ���Ũ��ˮ����������һ������������森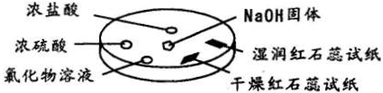 �±��ж�ʵ�����������Ľ��Ͳ���ȷ���ǣ�������
|
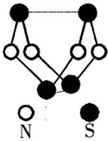 ��2010?�Ϸ���ģ���ִ�����ѧ����һ����������о�����Ϊ��Ծ������֮һ������ͼ���Ѿ��ϳɵ�����������һ��������ķ��ӽṹ������˵��������ǣ�������
��2010?�Ϸ���ģ���ִ�����ѧ����һ����������о�����Ϊ��Ծ������֮һ������ͼ���Ѿ��ϳɵ�����������һ��������ķ��ӽṹ������˵��������ǣ�������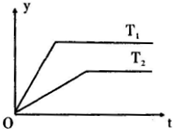 ��2010?�Ϸ���ģ����һ�ܱ������г���4mol SO2��һ����O2��������Ӧ��2SO2��g��+O2��g��?2SO3��g����H=һ196.6kJ?mol-1��������˵����ȷ���ǣ�������
��2010?�Ϸ���ģ����һ�ܱ������г���4mol SO2��һ����O2��������Ӧ��2SO2��g��+O2��g��?2SO3��g����H=һ196.6kJ?mol-1��������˵����ȷ���ǣ�������