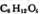��Ŀ����
�����йػ�ѧʵ��Ļ�����������������ȷ���� ������ţ���
A���ü�ʽ�ζ�����ȡ20.00mL 0.10mol/L KMnO4��Һ��
B����ȥ������Һ�л��е�Na2SO4���������ķ�����
C���ò�����պȡ����Һ����������ˮ��ʪ��pH��ֽ�ϣ��۲���ɫ�仯�������ɫ���Ƚϲ�ø���Һ��pH��
D������к͵ζ�ʵ���У������ζ���Һ��ʱ����С�ĵ��ڡ�0���̶ȣ����������ټ�Һ����0���̶ȣ�
E������һ�����ʵ���Ũ�ȵ���Һʱ��������ˮ������ƿϴ�Ӹɾ���������ֱ��ʹ�ã�
F������ͭ������ᾧˮ�����IJⶨʵ���У����Ⱥ����õĹ����ڿ�������ȴ�������������ʵ����ƫ��
A���ü�ʽ�ζ�����ȡ20.00mL 0.10mol/L KMnO4��Һ��
B����ȥ������Һ�л��е�Na2SO4���������ķ�����
C���ò�����պȡ����Һ����������ˮ��ʪ��pH��ֽ�ϣ��۲���ɫ�仯�������ɫ���Ƚϲ�ø���Һ��pH��
D������к͵ζ�ʵ���У������ζ���Һ��ʱ����С�ĵ��ڡ�0���̶ȣ����������ټ�Һ����0���̶ȣ�
E������һ�����ʵ���Ũ�ȵ���Һʱ��������ˮ������ƿϴ�Ӹɾ���������ֱ��ʹ�ã�
F������ͭ������ᾧˮ�����IJⶨʵ���У����Ⱥ����õĹ����ڿ�������ȴ�������������ʵ����ƫ��
������A�����������������
B��������ҺΪ�����ɢϵ����������Ĥ��
C��pH��ֽ����ʪ��
D�������ζ���Һ������ڡ�0���̶����µ�ijһ�̶ȣ�
E������ƿ����Ҫ���
F���ڿ�������ȴ�����������ˮ��
B��������ҺΪ�����ɢϵ����������Ĥ��
C��pH��ֽ����ʪ��
D�������ζ���Һ������ڡ�0���̶����µ�ijһ�̶ȣ�
E������ƿ����Ҫ���
F���ڿ�������ȴ�����������ˮ��
����⣺A�����������������Ӧ����ʽ�ζ�����ȡ20.00mL 0.10mol/L KMnO4��Һ����A����
B��������ҺΪ�����ɢϵ����������Ĥ������Һ���ԣ����ȥ������Һ�л��е�Na2SO4���������ķ�������B��ȷ��
C��pH��ֽ����ʪ������ܲ�������C����
D�������ζ���Һ������ڡ�0���̶����µ�ijһ�̶ȣ��������Ϊ0����C����
E������ƿ����Ҫ�����ʵ����Ӱ�죬��E��ȷ��
F���ڿ�������ȴ�����������ˮ��Ӧ�ڸ���������ȴ��������F����
�ʴ�Ϊ��BE��
B��������ҺΪ�����ɢϵ����������Ĥ������Һ���ԣ����ȥ������Һ�л��е�Na2SO4���������ķ�������B��ȷ��
C��pH��ֽ����ʪ������ܲ�������C����
D�������ζ���Һ������ڡ�0���̶����µ�ijһ�̶ȣ��������Ϊ0����C����
E������ƿ����Ҫ�����ʵ����Ӱ�죬��E��ȷ��
F���ڿ�������ȴ�����������ˮ��Ӧ�ڸ���������ȴ��������F����
�ʴ�Ϊ��BE��
���������⿼�黯ѧʵ�鷽�������ۣ��漰�к͵ζ��������ᴿ��pH�ⶨ����Һ���ơ��ᾧˮ�IJⶨ�ȣ����ػ���֪ʶ�Ŀ��飬ע��ʵ���е�ϸ�ں�������ʹ�ã���Ŀ�ѶȲ���ѡ��FΪ�״��㣮

��ϰ��ϵ�д�
 �������¿��ÿ�ʱ��ҵϵ�д�
�������¿��ÿ�ʱ��ҵϵ�д� Ӣ�żƻ�ͬ����ʱ��Чѵ��ϵ�д�
Ӣ�żƻ�ͬ����ʱ��Чѵ��ϵ�д�
�����Ŀ
�����йػ�ѧ�����ʾ��ȷ���ǣ�������
��CSO�ĵ���ʽ��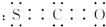
�ڶ��������ӵĽṹ��ʽ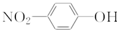
��Cl-�Ľṹʾ��ͼ��
�ܼ�����ӵı���ģ�ͣ�
�������ǵ�ʵ��ʽ��CH2O
��ԭ�Ӻ�����20�����ӵ���ԭ�ӣ�
Cl
��HCO3-��ˮ�ⷽ��ʽ��HCO3-+H2O?CO32-+H3O+��
��CSO�ĵ���ʽ��
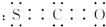
�ڶ��������ӵĽṹ��ʽ

��Cl-�Ľṹʾ��ͼ��
�ܼ�����ӵı���ģ�ͣ�

�������ǵ�ʵ��ʽ��CH2O
��ԭ�Ӻ�����20�����ӵ���ԭ�ӣ�
20 17 |
��HCO3-��ˮ�ⷽ��ʽ��HCO3-+H2O?CO32-+H3O+��
| A���٢ܢ� | B���ڢۢܢ� |
| C���ۢݢޢ� | D��ȫ����ȷ |
 �ڶ��������ӵĽṹ��ʽ��
�ڶ��������ӵĽṹ��ʽ��
 �ܼ�����ӵı���ģ�ͣ�
�ܼ�����ӵı���ģ�ͣ�
 CO32����H3O��
CO32����H3O��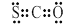 �ڶ��������ӵĽṹ��ʽ��
�ڶ��������ӵĽṹ��ʽ��
 �ܼ�����ӵı���ģ�ͣ�
�ܼ�����ӵı���ģ�ͣ�
 CO32����H3O��
CO32����H3O��