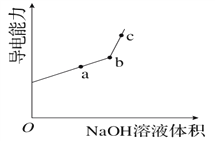
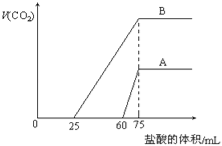
��Ŀ����
����Ŀ����ͼ��ʾ��ͼ���ʾ����ϸ����Ԫ�ء������a��b��c��d������ͬ��С�������ʣ�A��B��E��F������ͬ�Ĵ�������ʣ�������ش��������⣺
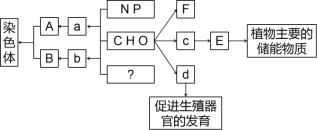
��1���ڶ���ϸ���ڣ�������E�����������������__________����FΪֲ��ϸ��ϸ���ڵ���Ҫ�ɷ֣������ĺϳ���Ҫ��������˿���ѵ�________�ڡ�
��2������d��__________�����̴������ڹ̴������ʡ�
��3��������Ԫ��Ϊ________���ɶ��b�����γ�B�Ĺ��̱���Ϊ_____________��
��4����ͼΪȾɫ��ṹ�е�һ���֣����뽫��������С�����Ի�ѧ�����������������ӵ�����Ϊ_______________��
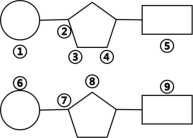
���𰸡���ԭ ĩ ά����D N����Ԫ�أ� ��ˮ���� �ۢ�
��������
������⣬������ͼ��Ⱦɫ����DNA�͵�������ɣ������DNA������Ԫ����ɣ���A��DNA��aΪ���������B�ǵ����ʣ�b�ǰ����ᣬ��������Ԫ����N��E��ֲ��ϸ���Ĵ������ʣ�������ǵ��ۣ�e�������ǣ�d�ܴٽ���ֳ���ٵķ��������Լ��ء�
��1���ɷ�����֪��E�ǵ��ۣ�������ɵ�λe�������ǣ�����ϸ������������Ƶ���������ԭ��ֲ��ϸ��ϸ���ڵĺϳ���Ҫ�߶������йأ���������˿���ѵ�ĩ�ڡ�
��2������d���Լ��أ�����ά����D�͵��̴��ȶ����ڹ̴������ʡ�
��3�����ǰ��ķ�����֪��B�ǵ����ʣ�b�ǰ����ᣬ����Ԫ��ΪN���ɶ�������������γɵ����ʵĹ��̱���Ϊ��ˮ���ϡ�
��4����ͼΪ����DNA�Ļ�������Ľṹ��ͼ���������������ͼ��DNA����������������������������������֮���γ�3,5-������������ӣ���ͼ�Тۢ������γ������������

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�