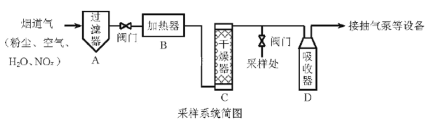
��Ŀ����
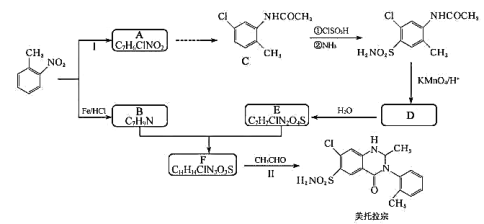
����Ŀ��ij�о�С�����������ױ�Ϊ��ʼԭ�ϣ�������·�ߺϳ�����ҩ�������ڡ�
��֪��
R-COOH+ 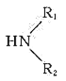
![]()
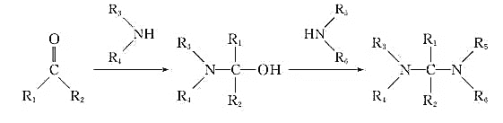
��ش�
(1)����˵����ȷ����________��
A ��Ӧ����Լ���������![]() ���� B ������C�ܷ���ˮ�ⷴӦ
���� B ������C�ܷ���ˮ�ⷴӦ
C ��Ӧ���漰���ӳɷ�Ӧ��ȡ����Ӧ D �������ڵķ���ʽ��![]()
(2)д��������D�Ľṹ��ʽ_________��
(3)д��![]() �Ļ�ѧ����ʽ____________��
�Ļ�ѧ����ʽ____________��
(4)�����A����ϩΪԭ�Ϻϳ�C��·��(������ͼ��ʾ�����Լ���ѡ)____��
(5)д��������Aͬʱ��������������ͬ���칹��Ľṹ��ʽ_____��
![]() ��
��![]() ���������ٷ����й���4����ԭ�ӣ����л��ϵ���2�֣�����̼��˫������������
���������ٷ����й���4����ԭ�ӣ����л��ϵ���2�֣�����̼��˫������������![]() ��
��
���𰸡�BC 
 +H2O
+H2O 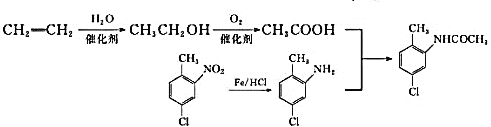
��������
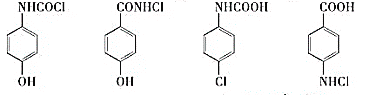
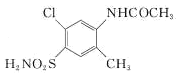
����������ױ���C�Ľṹ��ʽ�Լ�B�ķ���ʽ�������Ʋ��A�Ľṹ��ʽΪ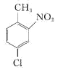 ��B�Ľṹ��ʽΪ
��B�Ľṹ��ʽΪ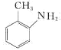 ��
�� �����Եĸ��������Һ����ΪD��D�Ľṹ��ʽΪ
�����Եĸ��������Һ����ΪD��D�Ľṹ��ʽΪ ��D�����к����ļ���һ��������ˮ������E�����E�ķ���ʽ��֪��E�Ľṹ��ʽΪ
��D�����к����ļ���һ��������ˮ������E�����E�ķ���ʽ��֪��E�Ľṹ��ʽΪ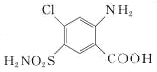 �����E�Ľṹ��ʽ���������ڵĽṹ��ʽ��F�ķ���ʽ�������֪��֪��F�Ľṹ��ʽΪ
�����E�Ľṹ��ʽ���������ڵĽṹ��ʽ��F�ķ���ʽ�������֪��֪��F�Ľṹ��ʽΪ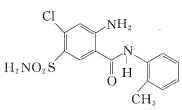 ��
��
��1��A����![]() ��
�� �������λ�ı����ϵ���ԭ�ӱ���ԭ�Ӵ��棬�ʷ�Ӧ���Լ���Һ�ȡ�FeCl3������FeCl3��������A����
�������λ�ı����ϵ���ԭ�ӱ���ԭ�Ӵ��棬�ʷ�Ӧ���Լ���Һ�ȡ�FeCl3������FeCl3��������A����
B��������C�к����ļ������Է���ˮ�⣬B��ȷ��
C�����F�Ľṹ��ʽ��CH3CHO���������ڵĽṹ��ʽ�Լ���֪��������Ӧ���漰���ӳɷ�Ӧ��ȡ����Ӧ��C��ȷ��
D���������ڵķ���ʽΪC16H16ClN3O3S��D����
��ѡBC��
��2���ɷ���֪������D�Ľṹ��ʽΪ ��
��
��3���ɷ�����֪��B�Ľṹ��ʽΪ ��E�Ľṹ��ʽΪ
��E�Ľṹ��ʽΪ ��F�Ľṹ��ʽΪ
��F�Ľṹ��ʽΪ ��B��E����ȡ����Ӧ����F���䷴Ӧ����ʽΪ��
��B��E����ȡ����Ӧ����F���䷴Ӧ����ʽΪ�� +
+ ��
�� +H2O��
+H2O��
��4����ϩ��ˮ�����ӳɷ�Ӧ�����Ҵ����Ҵ�������Ϊ���ᣬ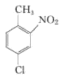 ��һ�������±���ԭΪ
��һ�������±���ԭΪ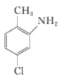 ��������
�������� ����ȡ����Ӧ����
����ȡ����Ӧ����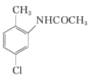 ������ͼΪ
������ͼΪ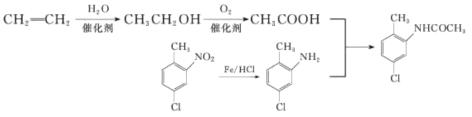 ��
��
��5����������4����ԭ�Ӳ��ұ�������2�֣������к���̼��˫����������CHO�͵��������ʷ����б����Ϻ�������ȡ�������������������Ƿֱ�Ϊ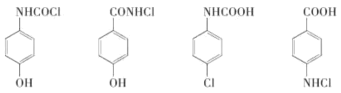 ��
��