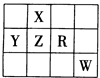
��Ŀ����
A��B��C��D��E��F��ԭ������������������ֳ���Ԫ�ء�E�ĵ�����C2��ȼ��
�IJ����ʹƷ����Һ��ɫ��F��CԪ���γɵĻ�����F3C4���д��ԡ�A�ĵ�����C2��ȼ��
������AC��AC2�������塣D�ĵ�����һ�ֽ������ý�����AC2�о���ȼ�����ɺڡ�����
�ֹ��塣��ش��������⣺
��B�ĵ��ʷ��ӵĵ���ʽΪ ��F3C4�Ļ�ѧʽΪ ��
FԪ�������ڱ��е�λ����_____________��
��д��D��AC2����ȼ�����ɺڰ����ֹ���Ļ�ѧ��Ӧ����ʽ
��A��B��C�γɵ�10�����⻯���У�A��B���⻯��е�ϵ͵��ǣ�д��ѧʽ��____________��
B��C���⻯����ӽ��H��������ǿ���ǣ�д��ѧʽ��______________����һ������
����ʽ����֤��____________________________��
��EC2����ͨ��BaCl2��HNO3�Ļ����Һ�����ɰ�ɫ��������ɫ����BC���йط�Ӧ����
�ӷ���ʽΪ__________________________________________���ɴ˿�֪BC2��EC2��
ԭ�Խ�ǿ���ǣ�д��ѧʽ��______________________________��
�IJ����ʹƷ����Һ��ɫ��F��CԪ���γɵĻ�����F3C4���д��ԡ�A�ĵ�����C2��ȼ��
������AC��AC2�������塣D�ĵ�����һ�ֽ������ý�����AC2�о���ȼ�����ɺڡ�����
�ֹ��塣��ش��������⣺
��B�ĵ��ʷ��ӵĵ���ʽΪ ��F3C4�Ļ�ѧʽΪ ��
FԪ�������ڱ��е�λ����_____________��
��д��D��AC2����ȼ�����ɺڰ����ֹ���Ļ�ѧ��Ӧ����ʽ
��A��B��C�γɵ�10�����⻯���У�A��B���⻯��е�ϵ͵��ǣ�д��ѧʽ��____________��
B��C���⻯����ӽ��H��������ǿ���ǣ�д��ѧʽ��______________����һ������
����ʽ����֤��____________________________��
��EC2����ͨ��BaCl2��HNO3�Ļ����Һ�����ɰ�ɫ��������ɫ����BC���йط�Ӧ����
�ӷ���ʽΪ__________________________________________���ɴ˿�֪BC2��EC2��
ԭ�Խ�ǿ���ǣ�д��ѧʽ��______________________________��
��10�֣�(4)��2�֣�����ÿ��1�֣���1�� ��Fe3O4 ���������ڵڢ�����
��Fe3O4 ���������ڵڢ�����
��2��2Mg + CO2 2MgO + C ��3��CH4 ��NH3 ��NH3 + H3+O = NH4+ + H2O
2MgO + C ��3��CH4 ��NH3 ��NH3 + H3+O = NH4+ + H2O
��4�� 3SO2 + 2NO3��+ 2H2O = 3SO42-+ 2NO�� + 4H+ ��2�֣� ��SO2
 ��Fe3O4 ���������ڵڢ�����
��Fe3O4 ���������ڵڢ�������2��2Mg + CO2
 2MgO + C ��3��CH4 ��NH3 ��NH3 + H3+O = NH4+ + H2O
2MgO + C ��3��CH4 ��NH3 ��NH3 + H3+O = NH4+ + H2O��4�� 3SO2 + 2NO3��+ 2H2O = 3SO42-+ 2NO�� + 4H+ ��2�֣� ��SO2
���������E�ĵ�����C2��ȼ�յIJ����ʹƷ����Һ��ɫ����û�������SO2������E��S��C��O��F��CԪ���γɵĻ�����F3C4���д��ԣ�����F������A�ĵ�����C2��ȼ�տ�����AC��AC2�������壬��A��̼Ԫ�أ�����B�ǵ�Ԫ�ء�D�ĵ�����һ�ֽ������ý�����AC2�о���ȼ�����ɺڡ������ֹ��壬����D��þ��
��B�ĵ��ʷ��ӵĵ���ʽΪ
 ��F3C4�Ļ�ѧʽΪFe3O4 ����Ԫ�������ڱ��е�λ���ǵ������ڵڢ����塣
��F3C4�Ļ�ѧʽΪFe3O4 ����Ԫ�������ڱ��е�λ���ǵ������ڵڢ����塣��þ��CO2�о���ȼ�����ɺڰ����ֹ���Ļ�ѧ��Ӧ����ʽΪ2Mg + CO2
 2MgO + C����A��B��C�γɵ�10�����⻯���У����ڰ������Ӽ�������������A��B���⻯��е�ϵ͵���CH4�������Ǽ������壬����B��C���⻯����ӽ��H��������ǿ����NH3���йط�Ӧ�ķ���ʽ��NH3 + H3+O = NH4+ + H2O��
2MgO + C����A��B��C�γɵ�10�����⻯���У����ڰ������Ӽ�������������A��B���⻯��е�ϵ͵���CH4�������Ǽ������壬����B��C���⻯����ӽ��H��������ǿ����NH3���йط�Ӧ�ķ���ʽ��NH3 + H3+O = NH4+ + H2O����SO2���л�ԭ�ԣ�ѧ�����������ԣ�����EC2����ͨ��BaCl2��HNO3�Ļ����Һ�з�����Ӧ�ĵ��뷽��ʽΪ3SO2 + 2NO3��+ 2H2O��3SO42-+ 2NO�� + 4H+�����ڻ�ԭ���Ļ�ԭ��ǿ�ڻ�ԭ����ģ����Ը��ݷ���ʽ��֪BC2��EC2��ԭ�Խ�ǿ����SO2��
�������������е��Ѷȵ����⣬Ҳ�Ǹ߿��еij������͡����������ǿ���������У����ض�ѧ�������������ͻ���֪ʶ�Ĺ��̡�������Ҫ���ԡ����ڱ���Ԫ�ص��ƶϡ�Ϊ���壬����ѧ����Ԫ�����ڱ�����Ϥ�̶ȼ���Ա��и�Ԫ�����ʺ���Ӧԭ�ӽṹ�������Եݱ���ɵ���ʶ�����ճ̶ȡ�������ѧ�������ʽṹ�����ʹ�ϵ�Լ�����Ԫ�������ɽ�����廯ѧ�����������

��ϰ��ϵ�д�
�����Ŀ