��Ŀ����
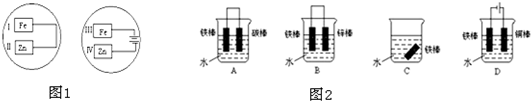
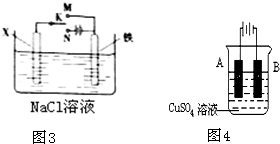
�� I����Zn��Fe��Cu��Ag���ֽ������±���װ�ý���ʵ�飬����ʵ�����������
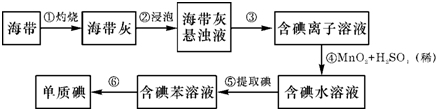
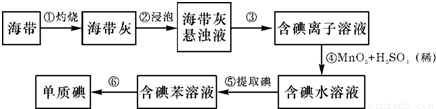
�� II��������������Ԫ��֮һ������ֲ���纣���������к��зḻ���Ե�������ʽ���ڵĵ�Ԫ�أ������к��зḻ�ĵ⣮Ϊ�˴Ӻ�������ȡ�⣬ij�о���ѧϰС����Ʋ�����������ʵ�飺
����д���пհף�
��1����������պ���ʱ������Ҫ���ż��⣬����Ҫ�õ���ʵ����������Ʒ��
A���ձ� B������ C�������� D�������� E���ƾ��� F�������� G��ʯ����
��2������۵�ʵ�����������
��3�������Ҳ������
��4��������У���ѡ���ñ�����ȡ�⣬����ѡ����Լ���
A���ƾ� B������ C�����Ȼ�̼ D������ E������ F��ֱ������
��5�������һ�ּ�����ȡ����ˮ��Һ���Ƿ��е��ʵ�ļ�����
װ �� |
 |
 |
 |
| �� �� | ����A�����ܽ� | C���������� | A����������� |
| ������Ӧʽ |
����д���пհף�

��1����������պ���ʱ������Ҫ���ż��⣬����Ҫ�õ���ʵ����������Ʒ��
BDE
BDE
������ĸ��ţ���A���ձ� B������ C�������� D�������� E���ƾ��� F�������� G��ʯ����
��2������۵�ʵ�����������
����
����
�������ʵ�������������ȡ��Һ
��ȡ��Һ
����3�������Ҳ������
Cl2
Cl2
���Լ����ƣ�������ͬ���ã�д���÷�Ӧ�����ӷ���ʽ2I-+Cl2=I2+2Cl-
2I-+Cl2=I2+2Cl-
����4��������У���ѡ���ñ�����ȡ�⣬����ѡ����Լ���
CEF
CEF
��A���ƾ� B������ C�����Ȼ�̼ D������ E������ F��ֱ������
��5�������һ�ּ�����ȡ����ˮ��Һ���Ƿ��е��ʵ�ļ�����
ȡ������ȡ����ˮ��Һ���Թ��У����뼸�ε�����Һ���۲��Ƿ������ɫ�����������˵�����е��ʵ⣩
ȡ������ȡ����ˮ��Һ���Թ��У����뼸�ε�����Һ���۲��Ƿ������ɫ�����������˵�����е��ʵ⣩
���������� I��ԭ�������������ԭ��Ӧ�����ݵ������Һ��д�缫����ʽ��
�� II��������Һ����Ϊ�����ͺ���������ҺӦѡ����˵ķ���������ˮת��Ϊ������л���Һ�����ö�±�ص����ܽ�����ǿ���л��ܼ��ѵ�ӵ�ˮ����ȡ��������Ӧ���е�ʵ���������ȡ������������Һͨ������Cl2��Ϊ�˽������������ɵ��ʵ⣬���ӷ���ʽΪ2I?+Cl2=I2+2Cl-�����õ��������л��ܼ���������������ȡ����ע�⣺ѡ�������ȡ����Լ����˶�I2��ǿ���ܽ���������������������ˮ�������ܽ��з�Һ���룮
�� II��������Һ����Ϊ�����ͺ���������ҺӦѡ����˵ķ���������ˮת��Ϊ������л���Һ�����ö�±�ص����ܽ�����ǿ���л��ܼ��ѵ�ӵ�ˮ����ȡ��������Ӧ���е�ʵ���������ȡ������������Һͨ������Cl2��Ϊ�˽������������ɵ��ʵ⣬���ӷ���ʽΪ2I?+Cl2=I2+2Cl-�����õ��������л��ܼ���������������ȡ����ע�⣺ѡ�������ȡ����Լ����˶�I2��ǿ���ܽ���������������������ˮ�������ܽ��з�Һ���룮
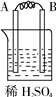
����⣺�� I��װ��1�У�����A�����ܽ⣬��ӦΪ������BΪ������������ӦΪ2H++2e-=H2����
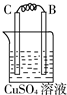
װ��2�У�C���������ӣ���˵��Cu2+�������õ��ӱ���ԭ���缫��ӦΪCu2++2e-=Cu��
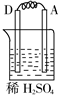
װ��3�У�A�������������˵��AΪԭ���������������ԭ��Ӧ�����������缫��ӦΪ2H++2e-=H2����
�ʴ�Ϊ��2H++2e-=H2����Cu2++2e-=Cu�� 2H++2e-=H2����
�� II�� ��1����������պ�����Ϊ����ļ��ȣ�Ӧ�������н��У���Ҫ�����������żܡ������ǡ������Լ��ƾ��Ƶ��������ʴ�Ϊ��BDE��
��2��������Һ����Ϊ�����ͺ���������ҺӦѡ����˵ķ���������ˮת��Ϊ������л���Һ�����ö�±�ص����ܽ�����ǿ���л��ܼ��ѵ�ӵ�ˮ����ȡ��������Ӧ���е�ʵ���������ȡ���ʴ�Ϊ�����ˣ���ȡ��Һ��
��3������������Һ�м�����������ͨ������Cl2��Ϊ�˽������������ɵ��ʵ⣬���ӷ���ʽΪ2I?+Cl2=I2+2Cl-��
�ʴ�Ϊ��Cl2��2I-+Cl2=I2+2Cl-��
��4�����õ��������л��ܼ���������������ȡ����ע�⣺ѡ�������ȡ����Լ����˶�I2��ǿ���ܽ���������������������ˮ�������ܽ��з�Һ���룬���������������Ȼ�̼�������ֱ�����͵ȣ��ʴ�Ϊ��CEF��
��5�����ݵ����������ɫ����ⵥ�ʵĴ��ڣ�ʵ�����Ϊȡ������ȡ����ˮ��Һ���Թ��У����뼸�ε�����Һ���۲��Ƿ������ɫ�����������˵�����е��ʵ⣩��
�ʴ�Ϊ��ȡ������ȡ����ˮ��Һ���Թ��У����뼸�ε�����Һ���۲��Ƿ������ɫ�����������˵�����е��ʵ⣩��
װ��2�У�C���������ӣ���˵��Cu2+�������õ��ӱ���ԭ���缫��ӦΪCu2++2e-=Cu��
װ��3�У�A�������������˵��AΪԭ���������������ԭ��Ӧ�����������缫��ӦΪ2H++2e-=H2����
�ʴ�Ϊ��2H++2e-=H2����Cu2++2e-=Cu�� 2H++2e-=H2����
�� II�� ��1����������պ�����Ϊ����ļ��ȣ�Ӧ�������н��У���Ҫ�����������żܡ������ǡ������Լ��ƾ��Ƶ��������ʴ�Ϊ��BDE��
��2��������Һ����Ϊ�����ͺ���������ҺӦѡ����˵ķ���������ˮת��Ϊ������л���Һ�����ö�±�ص����ܽ�����ǿ���л��ܼ��ѵ�ӵ�ˮ����ȡ��������Ӧ���е�ʵ���������ȡ���ʴ�Ϊ�����ˣ���ȡ��Һ��
��3������������Һ�м�����������ͨ������Cl2��Ϊ�˽������������ɵ��ʵ⣬���ӷ���ʽΪ2I?+Cl2=I2+2Cl-��
�ʴ�Ϊ��Cl2��2I-+Cl2=I2+2Cl-��
��4�����õ��������л��ܼ���������������ȡ����ע�⣺ѡ�������ȡ����Լ����˶�I2��ǿ���ܽ���������������������ˮ�������ܽ��з�Һ���룬���������������Ȼ�̼�������ֱ�����͵ȣ��ʴ�Ϊ��CEF��
��5�����ݵ����������ɫ����ⵥ�ʵĴ��ڣ�ʵ�����Ϊȡ������ȡ����ˮ��Һ���Թ��У����뼸�ε�����Һ���۲��Ƿ������ɫ�����������˵�����е��ʵ⣩��
�ʴ�Ϊ��ȡ������ȡ����ˮ��Һ���Թ��У����뼸�ε�����Һ���۲��Ƿ������ɫ�����������˵�����е��ʵ⣩��
���������⿼���Ϊ�ۺϣ��漰�绯ѧ�Լ��Ʊ�ʵ�鷽������ƣ���Ŀ�Ѷ��еȣ�ע�����ʵ�������Ҫ���ע�����

��ϰ��ϵ�д�
�����Ŀ
�� I����Zn��Fe��Cu��Ag���ֽ������±���װ�ý���ʵ�飬����ʵ�����������
�� II��������������Ԫ��֮һ������ֲ���纣���������к��зḻ���Ե�������ʽ���ڵĵ�Ԫ�أ������к��зḻ�ĵ⣮Ϊ�˴Ӻ�������ȡ�⣬ij�о���ѧϰС����Ʋ�����������ʵ�飺
����д���пհף�
��1����������պ���ʱ������Ҫ���ż��⣬����Ҫ�õ���ʵ����������Ʒ��______ ������ĸ��ţ���
A���ձ� B������ C�������� D�������� E���ƾ��� F�������� G��ʯ����
��2������۵�ʵ�����������______�������ʵ�����������______��
��3�������Ҳ������______���Լ����ƣ�������ͬ���ã�д���÷�Ӧ�����ӷ���ʽ______��
��4��������У���ѡ���ñ�����ȡ�⣬����ѡ����Լ���______��
A���ƾ� B������ C�����Ȼ�̼ D������ E������ F��ֱ������
��5�������һ�ּ�����ȡ����ˮ��Һ���Ƿ��е��ʵ�ļ�����______��
װ �� | 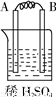 | 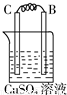 | 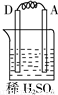 |
| �� �� | ����A�����ܽ� | C���������� | A����������� |
| ������Ӧʽ |
����д���пհף�

��1����������պ���ʱ������Ҫ���ż��⣬����Ҫ�õ���ʵ����������Ʒ��______ ������ĸ��ţ���
A���ձ� B������ C�������� D�������� E���ƾ��� F�������� G��ʯ����
��2������۵�ʵ�����������______�������ʵ�����������______��
��3�������Ҳ������______���Լ����ƣ�������ͬ���ã�д���÷�Ӧ�����ӷ���ʽ______��
��4��������У���ѡ���ñ�����ȡ�⣬����ѡ����Լ���______��
A���ƾ� B������ C�����Ȼ�̼ D������ E������ F��ֱ������
��5�������һ�ּ�����ȡ����ˮ��Һ���Ƿ��е��ʵ�ļ�����______��
 mol
mol