��Ŀ����
����12�֣�
��1����CO2Ϊ̼Դ��ȡ��̼�л���һֱ�ǻ�ѧ������о��ȵ㣬CO2������ȡ��̼���ķ�Ӧ���£�
��ӦI��CO2(g)+3H2(g)=CH3OH(g)+H2O(g) ��H=��49.0kJ/mol
��ӦII��2CO2(g)+6H2(g)=CH3CH2OH(g)+3H2O(g) ��H=��173.6kJ/mol
д����CH3OH(g)�ϳ�CH3CH2OH(g)�ķ�Ӧ���Ȼ�ѧ����ʽ_______________
��2��������أ�K2FeO4��������һ����Ҫ��������м�ǿ��������
�ٵ�ⷨ�ǹ�ҵ���Ʊ�K2FeO4��һ�ַ���������Ϊ�����������������Һ��Ȼ����������Һ�м���KOH�����ʱ����������Ӧ����FeO42-���õ缫��ӦʽΪ_________________
����MnO2��Zn������ƣ�K2FeO4��ZnҲ������ɼ��Ե�أ�K2FeO4�ڵ�������������ϣ���缫��ӦʽΪFeO42-+3e-+4H2O=Fe(OH)3+5OH-����õ���ܷ�Ӧ�����ӷ���ʽΪ__________________
��3��amol FeS��bmol FeOͶ�뵽VL��C mol/L��������Һ�г�ַ�Ӧ����NO���壬���ó�����Һ�ɷֿɿ�����Fe(NO3)3��H2SO4�Ļ��Һ����Ӧ��δ����ԭ���������Ϊ_____
�٣�a+b����63g �ڣ�a+b����189g �ۣ�a+b��mol ��VC��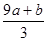 mol
mol
��1��2CH3OH(g)=CH3CH2OH(g)+H2O(g) ��H= -75.6kJ/mol
��2����Fe��6e- +8OH- = FeO42- + 4H2O
��3Zn + 2FeO42- + 8H2O = 2Fe(OH)3 + 3Zn(OH)2 + 4OH-
��3���ڢ�
��������
���������(1)��˹���ɣ����ܻ�ѧ��Ӧ��һ����ɻ�ּ�����ɣ��䷴Ӧ����ͬ������
2CH3OH(g)+2H2O(g) =2CO2(g)+6H2(g) ��H=��98.0kJ/mol ��
2CO2(g)+6H2(g)=CH3CH2OH(g)+3H2O(g) ��H=��173.6kJ/mol ��
��+�ڵ�2CH3OH(g)=CH3CH2OH(g)+H2O(g) ��H= -75.6kJ/mol
��2�������У�����������ӵĵ缫��������������������Ӧ���������ӵĵ缫����������������ԭ��Ӧ��
�ٴ�������Ϊ�������������������Һ������FeO42-���ʵ缫����ʽΪ
Fe��6e- +8OH- = FeO42- + 4H2O
����K2FeO4��Zn���Ե���У������õ��ӱ���ԭ����缫��ӦʽΪ
FeO42-��3e-��4H2O��Fe��OH��3��5OH-������Zn��������
��缫��ӦʽΪ��Zn��2e-��2OH-��Zn��OH��2����������ʧ�������غ��ϲ��ɵ��ܷ�Ӧʽ��2FeO42-��8H2O��3Zn��2Fe��OH��3��3Zn��OH��2��4OH-��
��3����Ӧ���������������ã�һ��������������ԭ��NO���塣���������������������������һ������������ϼ�û�仯��û�б���ԭ�����Ը��ݷ�Ӧǰ����������������֪����Ӧ����������������һ����֪����û����ԭ�������������Ӧǰ��a mol FeS��b mol FeO ��������ԭ���غ�֪����Ӧ��Fe(NO3)3�����ʵ����ǣ�a+b��mol����ô��������������ʵ�����3��a+b��mol������3��a+b��mol����û�б���ԭ����������
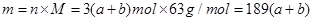 ������ȷ��
������ȷ��
������Դӵ�ʧ���ӽǶȿ��ǡ�
FeS������Ϊ�����������ᣬ������1�ۣ�������8�ۣ�����a mol FeS��Ӧ��ʧȥ����9amol��FeO������Ϊ��������������1�ۣ�����b mol FeO ��Ӧ��ʧȥ����bmol��
ʧȥ�ĵ��ӱ�������õ����������ԭ��NO����Ԫ�ػ��ϼ۽���3�ۣ����ݵ�ʧ�����غ�֪��������ԭ����������ʵ�����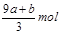 mol����Ϊ����������n==CVmol������δ����ԭ����������ʵ�����
mol����Ϊ����������n==CVmol������δ����ԭ����������ʵ�����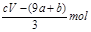 ���ܴ���ȷ��
���ܴ���ȷ��
���㣺��˹���ɡ�����ԭ����������������á�
���������⿼��ѧ���Ը�˹���ɵ��������գ��ڴ��������Ҫע����ŵ���д�Լ���Ч����
�ı������⣻����ԭ����Ҫ��ѧ��������صĵ缫����ʽ���ܵķ���ʽ������С�⣬��Ҫ
ѧ�����������ڷ�Ӧ�е�Ӧ�á�

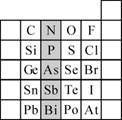
 ������ϵ�д�
������ϵ�д� �±�Сѧ��Ԫ�Բ���ϵ�д�
�±�Сѧ��Ԫ�Բ���ϵ�д�
 ��ɫ
��ɫ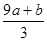 mol
mol