��Ŀ����
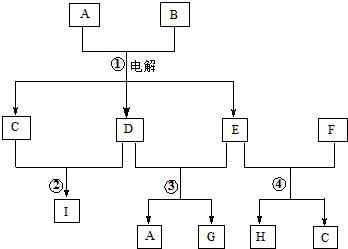
9��ͼ1Ϊ�������֮���ת����ϵ�����мס���Ϊ�����г����Ľ������ʣ��������ڳ�����Ϊ��̬�ǽ������ʣ�A��FΪ�����ˮ������ȥ������ش�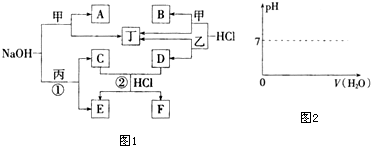
��1����ɱ���Ԫ����Ԫ�����ڱ��е�λ��Ϊ�������ڵ�VIIA�壻���÷�Ӧ�٣���ҵ�ϳ��Ʊ�Ư��Һ�����Ʒ���ƣ���
��2��C�ĵ���ʽΪ
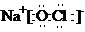 ����Ӧ�ڵ����ӷ���ʽΪClO-+2Fe2++2H+=Cl-+2Fe3++H2O��
����Ӧ�ڵ����ӷ���ʽΪClO-+2Fe2++2H+=Cl-+2Fe3++H2O����3����A��B��ˮ��Һ��ϣ���Ӧ�����ӷ���ʽΪAl3++3AlO2-+6H2O=4Al��OH��3����
��4����B��ϡ��Һ��ˮϡ�ͣ���ͼ2�л�����ҺpH���ˮ����ı仯�������ߣ�
��5����ҵ��ұ�������Ļ�ѧ����ʽΪ2Al2O3�����ڣ�$\frac{\underline{\;\;\;���\;\;\;}}{����ʯ}$4Al+3O2����
��6���������ʵ�����A��NaOH�������ˮ������Һ�и����ӵ����ʵ���Ũ���ɴ�С��˳��Ϊc��Na+����c��OH-����c��AlO2-����c��H+��_��
��7������F�������ӵķ���ΪȡF�������Թ��У���ˮ�ܽ⣬�����еμ�KSCN��Һ������Һ��죬��Fe3+��
���� ת��ͼ�з�����֪���dz����������Ժ�����������Һ��Ӧ��˵����ΪAl���ס���Ϊ�����г����Ľ������ʣ���ΪFe���ס��Һ�HCl��Ӧ���������ͽ������Σ��ж϶�ΪH2��AΪNaAlO2��BΪAlCl3��DΪFeCl2���������ڳ�����Ϊ��̬�ǽ������ʣ���������������Һ��Ӧ˵��Ϊ��ΪCl2������������������Һ��Ӧ�����Ȼ��ơ��������ƺ�ˮ��CΪNaClO��EΪNaCl���������ƺ��Ȼ�������������Һ�з���������ԭ��Ӧ�����Ȼ��ƺ��Ȼ�����FΪFeCl3��
��1����Ϊ��Ԫ�أ��������ڵڢ���A�壬��Ӧ����Ư��Һ���Ʊ���
��2��CΪ��������Ϊ���ӻ������Ӧ������������������Һ�б����������������Ϊ�����ӣ�
��3�������Ӻ�ƫ���������˫ˮ����������������
��4��BΪ�Ȼ�����ˮϡ����ҺPH����
��5�������������������ʯ������õ���������
��6���������ʵ�����NaAlO2��NaOH�������ˮ��ƫ���������ˮ����Һ�Լ��ԣ�
��7�����������ӵļ��鷽�����ʵ�飬����������KSCN��Һ���ɫ��
��� �⣺��1����Ϊ��Ԫ�أ��������ڵڢ���A�壬��Ӧ��������������������Һ��Ӧ���ɴ������ơ��Ȼ��ƺ�ˮ�����ҺΪƯ��Һ�ijɷ֣�
�ʴ�Ϊ���������ڵ�VIIA�壬Ư��Һ��
��2��CΪ��������Ϊ���ӻ�������������������ԭ�Ӻ���ԭ���γɹ��ۼ�������ʽΪ��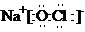
�ʴ�Ϊ��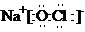
��3����A��B��ˮ��Һ����������Ӻ�ƫ���������˫ˮ������������������Ӧ�����ӷ���ʽΪ��Al3++3AlO2-+6H2O=4Al��OH��3����
�ʴ�Ϊ��Al3++3AlO2-+6H2O=4Al��OH��3����
��4��BΪ�Ȼ�����ˮϡ����ҺPH�������ܳ���PH=7��ͼ��Ϊ��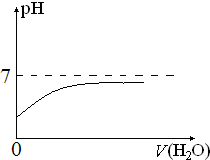 ��
��
�ʴ�Ϊ�� ��
��
��5�������������������ʯ������õ�����������Ӧ�Ļ�ѧ����ʽΪ��2Al2O3�����ڣ�$\frac{\underline{\;\;\;���\;\;\;}}{����ʯ}$4Al+3O2����
�ʴ�Ϊ��2Al2O3�����ڣ�$\frac{\underline{\;\;\;���\;\;\;}}{����ʯ}$4Al+3O2����
��6���������ʵ�����NaAlO2��NaOH�������ˮ��ƫ���������ˮ����Һ�Լ��ԣ�ʵ����Һ����Ũ�ȴ�СΪ��c��Na+����c��OH-����c��AlO2-����c��H+����
�ʴ�Ϊ��c��Na+����c��OH-����c��AlO2-����c��H+����
��7�����������ӵļ��鷽�����ʵ�飬����������KSCN��Һ���ɫ��ʵ�����Ϊ��ȡF�������Թ��У���ˮ�ܽ⣬�����еμ�KSCN��Һ������Һ��죬��Fe3+���ʴ�Ϊ��ȡF�������Թ��У���ˮ�ܽ⣬�����еμ�KSCN��Һ������Һ��죬��Fe3+��
���� ���⿼���������ת����ϵ���������ʵķ����жϣ���Ӧ����ͷ�Ӧ���̵ķ����ǽ���ؼ�����Ŀ�Ѷ��еȣ�

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�| A�� | Li2SO4������ˮ | B�� | LiOH��Li2CO3���ȶ����ѷֽ� | ||
| C�� | LiOH������ˮ | D�� | Li��N2��Ӧ������Li3N2 |
| A�� | Cu2Sֻ����ԭ����O2ֻ�������� | B�� | Cu2S��Cu�Ļ��ϼ�Ϊ+2�� | ||
| C�� | ÿ����1molSO2��ת�Ƶ���6mol | D�� | ÿ����2molCu��ת�Ƶ���2mol |
| A�� | ����Ӧ���Ũ�� | B�� | ��������ѹǿ | ||
| C�� | ���� | D�� | ʹ�ô��� |
| A�� | ����ˮ�������� | B�� | �������������Сһ�� | ||
| C�� | ����������䣬����N2 | D�� | ѹǿ���䣬����N2ʹ������� |
| A�� | �ױ��;ƾ� | B�� | �ױ��ͱ� | C�� | ��������ˮ | D�� | ���������� |
| A�� | ̼��������Һ��K+��SO42-��Cl-��H+ | |
| B�� | ����������c��H+��=10-12 mol•L-1����Һ��K+��SO42-��Cl-��Br- | |
| C�� | ��̪��Һ������Һ��Na+��Cl-��SO42-��Fe3+ | |
| D�� | ��ɫʯ����Һ������Һ��Fe2+��Mg2+��NO3-��Cl- |
| A�� | ʵ������Ũ������MnO2��Ӧ��Cl2��MnO2+2H++2Cl- $\frac{\underline{\;\;��\;\;}}{\;}$Cl2��+Mn2++H2O | |
| B�� | С�մ���Һ�м���������ʯ��ˮ��Ca2++2OH-+2HCO3-=CaCO3��+CO32-+2H2O | |
| C�� | ��Ca��ClO��2��Һ��ͨ��������SO2��Ca2++2ClO-+SO2+H2O=2HClO+CaSO3�� | |
| D�� | ̼�������Һ�ӵ������У�Ca��HCO3��2+2CH3COOH=Ca2++2CH3COO-+2CO2��+2H2O |
 ��
��