��Ŀ����
 ��ҵ�ϲ���SO2��N2��O2���������SO2������װ����ͼ��ʾ����Ӧ����װ�е�ĵ�����Һ��SO2��I2�����ķ�ӦΪ��N2��O2����I2��Ӧ����SO2+I2+2H2O�TH2SO4+2HI��
��ҵ�ϲ���SO2��N2��O2���������SO2������װ����ͼ��ʾ����Ӧ����װ�е�ĵ�����Һ��SO2��I2�����ķ�ӦΪ��N2��O2����I2��Ӧ����SO2+I2+2H2O�TH2SO4+2HI����1�����������뷴Ӧ�ܺ������������ӵ�ˮ���������
N2��O2
N2��O2
�������������ķ���ʽ������2����Ӧ������Һ��ɫ��ʧ��û�м�ʱֹͣͨ��������SO2����
ƫ��
ƫ��
���ƫ�ߡ�����ƫ�͡�������Ӱ�족������3����Ӧ���ڵĵ�ĵ�����ҺҲ������
���Ը��������Һ����ˮ
���Ը��������Һ����ˮ
���棨��д�������ƣ�����4��������װ�ø�Ϊ����ʵ��װ�ã��������⣬����ѡ�õ�����Ϊ
bceg��beg��ceg
bceg��beg��ceg
����������ţ���a���ձ� b���Թ� c�����ƿ d������ƿ e����Ͳ f�������� g��˫������
��������1��SO2��N2��O2���������뷴Ӧ�ܣ�SO2�����գ��������������ֵΪ�����������ӵ�ˮ�����������N2��O2�������
��2����Ӧ������Һ��ɫ��ʧʱ��SO2�뷴Ӧ����������I2ǡ�÷�Ӧ����û��ʱֹͣͨ������δ��Ӧ��SO2����Ҳ��ˮ�������ܣ�ʹN2��O2������ӣ����SO2�ĺ������ͣ�
��3����ĵ�����Һ�������ǵ����е�I2��ȫ��SO2��ԭʱ����Һ����ɫ������ɫ����ɫ��ָʾ�յ�ģ�������������SO2���巴Ӧ�����ʣ��ڴﵽ�յ�����ɫ�ı�ʱ��ɴ����ĵ�����Һ����KMnO4��Һ����ˮ�ȣ�
��4������ʵ��ԭ����ѡ����ʵ�ʵ��װ�ã�
��2����Ӧ������Һ��ɫ��ʧʱ��SO2�뷴Ӧ����������I2ǡ�÷�Ӧ����û��ʱֹͣͨ������δ��Ӧ��SO2����Ҳ��ˮ�������ܣ�ʹN2��O2������ӣ����SO2�ĺ������ͣ�
��3����ĵ�����Һ�������ǵ����е�I2��ȫ��SO2��ԭʱ����Һ����ɫ������ɫ����ɫ��ָʾ�յ�ģ�������������SO2���巴Ӧ�����ʣ��ڴﵽ�յ�����ɫ�ı�ʱ��ɴ����ĵ�����Һ����KMnO4��Һ����ˮ�ȣ�
��4������ʵ��ԭ����ѡ����ʵ�ʵ��װ�ã�
����⣺��1�����������뷴Ӧ���У�����SO2��I2������Ӧ��SO2+I2+2H2O�TH2SO4+2HI���������壬ʣ����������N2��O2�������������ӵ�ˮ���������N2��O2���������
�ʴ�Ϊ��N2��O2�������
��2�����ݷ�Ӧ��SO2+I2+2H2O�TH2SO4+2HI��������յ�SO2�������V��SO2��=Vm��n��I2����SO2������ٷֺ����գ�SO2��=
��ʽ��V��SO2����������n��I2�������ʵ��������ģ�����û�м�ʱֹͣͨ�����ͻ�ʹ��V��������壩������գ�SO2��ƫ�ͣ�
�ʴ�Ϊ��ƫ�ͣ�
��3����Ӧ���ڵĵ�ĵ�����Һ������������SO2����ɫ��Һ���棬��ѧ�����ҿ��õ�����ˮ����������Ȼ�̼��Һ����������������Һ����Ϊ�Ϻ�ɫ������ص���Һͨ���������ʱ�ᷢ��5SO2+2H2O+KMnO4�TK2SO4+2H2SO4+2MnSO4����ɫ��Һ���ķ�Ӧ����Һ�������Ϻ�ɫ�����ɫ����ˮ����������Ȼ�̼��Һ������SO2+Br2+2H2O�TH2SO4+2HBr�������ɫ������Ҳ�ɲ��ø��������Һ�����ĵ�����Һ��ɸ�ʵ�飬
�ʴ�Ϊ�����Ը��������Һ����ˮ��
��4��������װ�ø�Ϊ����ʵ��װ�ã��������⣬����ѡ�õ������Թܡ����ƿ����Ͳ��˫�����������Թܡ���Ͳ��˫������
�ʴ�Ϊ��bceg��beg��
�ʴ�Ϊ��N2��O2�������
��2�����ݷ�Ӧ��SO2+I2+2H2O�TH2SO4+2HI��������յ�SO2�������V��SO2��=Vm��n��I2����SO2������ٷֺ����գ�SO2��=
| ����������� |
| ����������� |
�ʴ�Ϊ��ƫ�ͣ�
��3����Ӧ���ڵĵ�ĵ�����Һ������������SO2����ɫ��Һ���棬��ѧ�����ҿ��õ�����ˮ����������Ȼ�̼��Һ����������������Һ����Ϊ�Ϻ�ɫ������ص���Һͨ���������ʱ�ᷢ��5SO2+2H2O+KMnO4�TK2SO4+2H2SO4+2MnSO4����ɫ��Һ���ķ�Ӧ����Һ�������Ϻ�ɫ�����ɫ����ˮ����������Ȼ�̼��Һ������SO2+Br2+2H2O�TH2SO4+2HBr�������ɫ������Ҳ�ɲ��ø��������Һ�����ĵ�����Һ��ɸ�ʵ�飬
�ʴ�Ϊ�����Ը��������Һ����ˮ��
��4��������װ�ø�Ϊ����ʵ��װ�ã��������⣬����ѡ�õ������Թܡ����ƿ����Ͳ��˫�����������Թܡ���Ͳ��˫������
�ʴ�Ϊ��bceg��beg��
���������⿼����Ƕ�����������ʡ�ʵ�������жϡ�ָʾ����ѡ���ѵ����ڣ�2��SO2��������������Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
 ������״Ԫ���Ծ�ϵ�д�
������״Ԫ���Ծ�ϵ�д�
�����Ŀ
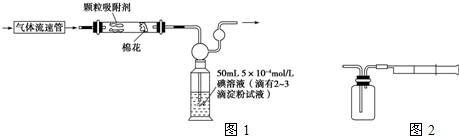






 ��ͬѧ���������ͼ��ʾ��ʵ��װ�ã��ⶨij�ؿ�����SO2�ĺ�����
��ͬѧ���������ͼ��ʾ��ʵ��װ�ã��ⶨij�ؿ�����SO2�ĺ����� ����ͬѧ��������ͼ����װ�òⶨ�����е�SO2������ȷ��ȡһ�������5��10-4mol/L�ĵ���Һ��ע����ͼ��ʾ���ƿ�У���2��3�ε���ָʾ������ʱ��Һ����ɫ����ָ���IJⶨ�ص������ÿ�γ���100mL��ֱ����Һ����ɫȫ���ʾ�Ϊֹ����¼����������n����
����ͬѧ��������ͼ����װ�òⶨ�����е�SO2������ȷ��ȡһ�������5��10-4mol/L�ĵ���Һ��ע����ͼ��ʾ���ƿ�У���2��3�ε���ָʾ������ʱ��Һ����ɫ����ָ���IJⶨ�ص������ÿ�γ���100mL��ֱ����Һ����ɫȫ���ʾ�Ϊֹ����¼����������n����