��Ŀ����
����Ŀ��һ�������£����淴Ӧ��ƽ�ⳣ��������ƽ��Ũ�ȼ��㣬Ҳ������ƽ���ѹ����ƽ��Ũ�ȼ��㣬��ѹ=��ѹ�����ʵ����������ں��º�ѹ�����£���ѹ���䣬��ƽ���ѹ����ƽ�ⳣ�������㣮����˵������ȷ���ǣ� ��
A.����C2H4 ��g��+H2O��g��?C2H5OH��g������һ�������´ﵽƽ��״̬ʱ����ϵ����ѹǿΪP������C2H4��g����H2O��g����C2H5OH��g����Ϊ2 mol�����÷�ѹ��ʾ��ƽ�ⳣ��Kp= ![]()
B.���º�ѹ�£���һ�ݻ��ɱ�������У�N2��g��+3H2��g��?2NH3��g���ﵽƽ��״̬ʱ��N2��H2��NH3��1mol������ʱ�ٳ���3mol N2 �� ��ƽ�������ƶ�
C.���º�ѹ�£���һ�ݻ��ɱ�������У���Ӧ2A��g��+B��g��?2C��g���ﵽƽ��ʱ��A��B�� C�����ʵ����ֱ�Ϊ4mol��2mol��4mol������ʱA��B��C������1 mol��ƽ�������ƶ�
D.����һ�������µ�ijһ���淴Ӧ����ƽ��Ũ�ȱ�ʾ��ƽ�ⳣ������ƽ���ѹ��ʾ��ƽ�ⳣ��������ֵ��ͬ����������ͬ����ֻ���¶��й�
���𰸡�B
���������⣺A����ϵ����ѹǿΪP������C2H4��g����H2O��g����C2H5OH��g����Ϊl mol����ѹP��C2H5OH��=P�� ![]() =
= ![]() ��P��C2H4��=P��
��P��C2H4��=P�� ![]() =
= ![]() ��P��H2O��=P��
��P��H2O��=P�� ![]() =
= ![]() ��ƽ�ⳣ��K=
��ƽ�ⳣ��K= ![]() =
= ![]() ����A��ȷ��
����A��ȷ��
B�����º�ѹ�£���һ�ݻ��ɱ�������У�N2��g��+3H2��g��2NH3��g���ﵽƽ��״̬ʱ��N2��H2��NH3��1mol����ƽ��ʱ�������Ϊ1L�����¶���ƽ�ⳣ��K= ![]() =1������ʱ�ٳ���3molN2 �� ���º�ѹ�����֮�ȵ����������ʵ���֮�ȣ����������=1L��
=1������ʱ�ٳ���3molN2 �� ���º�ѹ�����֮�ȵ����������ʵ���֮�ȣ����������=1L�� ![]() =2L��Qc=
=2L��Qc= ![]() =1=K��ƽ�ⲻ������B����
=1=K��ƽ�ⲻ������B����
C�����ݻ��ɱ������£��Ӷ�ƽ�����������ߵ����ʵ����Ը����ʵ�����Ũ��Ӱ��Ƕ�˼�����ڡ������롱�����ࡱʱ���൱��A��B��C�����ʵ�Ũ�ȶ�û�иı䣬ԭƽ��ʱA��B��C�����ʵ���֮��Ϊ2��1��2����������1mol��ʱ�൱���൱����ԭ���Ļ����ϼ�����B��ƽ�������ƶ�����C��ȷ��
D��ƽ�ⳣ�����¶ȱ仯��ƽ��Ũ�ȼ����ƽ���ѹ�������������ͬ������һ�������µ�ijһ���淴Ӧ����ƽ��Ũ�ȱ�ʾ�ļⳣ������ƽ���ѹ��ʾ��ƽ�ⳣ��������ֵ��ͬ����������ͬ����ֻ���¶��йأ���D��ȷ��
��ѡB��
�����㾫����������Ĺؼ��������⻯ѧƽ�ⳣ���ĺ�������֪ʶ������ָ��һ�������µĿ��淴Ӧ�����Ӧ���淴Ӧ��������ȣ���Ӧ������и���ֵ�Ũ�Ȳ����״̬���Լ��Ի�ѧƽ��ļ�������⣬�˽ⷴӦ��ת����=ת��Ũ�ȡ���ʼŨ�ȡ�100%=ת�����ʵ�������ʼ���ʵ�����100%����Ʒ�IJ���=ʵ�����ɲ�������ʵ����������Ͽɵõ���������ʵ�����100%��

 ͬ����ϰǿ����չϵ�д�
ͬ����ϰǿ����չϵ�д�����Ŀ����֪�������ữ�IJ��ᣨH2C2O4����Һ����KMnO4��Һ��Ӧ��ij��ѧС���о����֣�����MnSO4�ɶԸ÷�Ӧ������ã�Ϊ��һ���о��й����ضԸ÷�Ӧ���ʵ�Ӱ�죬̽�����£�
��1�������£�̽����ͬ�ij�ʼpH�Ͳ�����ҺŨ�ȶԷ�Ӧ���ʵ�Ӱ�죬�������ʵ�飬��A= �� C= �� E=
ʵ���� | �¶� | ��ʼpH | 0.1mol/L������Һ���/mL | 0.01mol/LKMnO4 | ����ˮ���/mL | �������ݣ����Һ��ɫʱ��/s�� |
�� | ���� | 1 | 20 | 50 | 30 | t1 |
�� | ���� | A | B | C | 30 | t2 |
�� | ���� | 2 | 40 | D | E | t3 |
��2���÷�Ӧ�����ӷ���ʽ ��
��3����t1��t2 �� �����ʵ��ٺ͢ڵõ��Ľ����� ��
��4��С��ͬѧ����ÿ��ʵ�鷴Ӧ������ʱ��ı仯������ͼ������t1��t2ʱ�������ʱ�����Ҫԭ������ǣ� 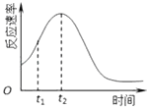
������
��5����ѧС���õζ����ⶨKMnO4��Һ���ʵ���Ũ�ȣ�ȡag���ᾧ�壨H2C2O42H2O��Ħ������126g/mol������ˮ���250mL��Һ��ȡ25.00mL��Һ������ƿ�У���������ϡH2SO4�ữ������KMnO4��Һ�ζ����յ㣬�ظ��ζ����Σ�ƽ������KMnO4��ҺVmL���ζ������յ�������ǣ���ʵ��������Ķ��������������������ƣ�����KMnO4��Һ�����ʵ���Ũ��Ϊmol/L��