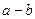��Ŀ����
��4�֣�A��B��C��D���ֶ�����Ԫ�أ�ԭ��������������AԪ��ԭ�������������Ǵ�����2����0.05 mol BԪ�ص����ӵõ���6.02��1022�����Ӻ�ԭΪ����ԭ�ӣ�0.4g B��������ǡ����100mL 0 .2mol/L��������ȫ��Ӧ��BԪ��ԭ�Ӻ�������������������ȡ�C���Ӻ�����Ӳ�����AԪ�ص����Ӻ�����Ӳ�����1�����γ����ӻ�����BC��D��A���γɻ��������գ�
.2mol/L��������ȫ��Ӧ��BԪ��ԭ�Ӻ�������������������ȡ�C���Ӻ�����Ӳ�����AԪ�ص����Ӻ�����Ӳ�����1�����γ����ӻ�����BC��D��A���γɻ��������գ�
��1��A��B ����Ԫ�ص�Ԫ�ط��ţ�B___________ ��C__________��
��2��D��A�γɻ��������ʽ��________�����ӿռ乹����________��
 .2mol/L��������ȫ��Ӧ��BԪ��ԭ�Ӻ�������������������ȡ�C���Ӻ�����Ӳ�����AԪ�ص����Ӻ�����Ӳ�����1�����γ����ӻ�����BC��D��A���γɻ��������գ�
.2mol/L��������ȫ��Ӧ��BԪ��ԭ�Ӻ�������������������ȡ�C���Ӻ�����Ӳ�����AԪ�ص����Ӻ�����Ӳ�����1�����γ����ӻ�����BC��D��A���γɻ��������գ���1��A��B ����Ԫ�ص�Ԫ�ط��ţ�B___________ ��C__________��
��2��D��A�γɻ��������ʽ��________�����ӿռ乹����________��
��1��Mg ��2�֣���S ��1�֣�дԪ�����Ʋ��÷֣�
��2��CCl4��1�֣��������壨1�֣�
��2��CCl4��1�֣��������壨1�֣�
��

��ϰ��ϵ�д�
�����Ŀ

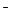 ��SCN
��SCN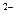 ��M+�ĵ��Ӳ�ṹ��ͬ����ԭ�������ıȽ�Ϊ��R>M
��M+�ĵ��Ӳ�ṹ��ͬ����ԭ�������ıȽ�Ϊ��R>M