̀âÄ¿ÄÚÈƯ
¡¾̀âÄ¿¡¿̀¼¼°Æ仯ºÏÎïÓëÈËÀàµÄÉú»î¡¢Éú²ú½ôĂÜÁªÏµ¡£
£¨1£©̉ÑÖª2g¼×ÍéÍêȫȼÉƠÉú³ÉÎȶ¨µÄÑơ»¯Îïʱ·Å³öQ kJµÄÈÈÁ¿,Đ´³ö±íʾ¼×ÍéȼÉƠÈȵÄÈÈ»¯Ñ§·½³̀ʽ£º___________
£¨2£©̉ÑÖª£ºC(s)£«H2O(g)=CO(g)£«H2(g) ¦¤H£½£«130 kJ¡¤mol£1£¬2C(s)£«O2(g)=2CO(g) ¦¤H£½£220 kJ¡¤mol£1¡£¶Ï¿ª1 mol H¡ªH¼ü¡¢O===O¼ü·Ö±đĐè̉ªÎüÊƠ436 kJ¡¢496 kJµÄÈÈÁ¿£¬Ộ¶Ï¿ª1 mol O¡ªH¼üĐè̉ªÎüÊƠµÄÈÈÁ¿Îª___________
A£®332 kJ B£®462 kJ C£®118 kJ D£®360 kJ
£¨3£©̉ÔCO2ºÍH2OΪÔÁÏÖƱ¸HCOOHºÍO2µÄÔµç³ØÔÀíÈçͼ£®
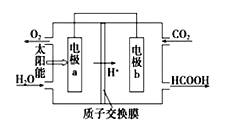
µç¼«aΪ_____(̀î¡°Ơư¡±»̣¡°¸º¡±)¼«£¬µç¼«b·¢ÉúµÄµç¼«·´Ó¦Ê½ÊÇ£º___________________£®
¡¾´đ°¸¡¿ CH4(g)+2O2(g)=CO2(g)+2H2O(l)¡÷H=-8QkJ/mol£¨ÏµÊư²»Äܱ䣩 B ¸º 2CO2+4e-+4H+=2HCOOH
¡¾½âÎö¡¿ÊỒâ·ÖÎö£º
£¨1£©ÓÉ2g¼×ÍéÍêȫȼÉƠÉú³ÉÎȶ¨µÄÑơ»¯Îïʱ·Å³öQ kJµÄÈÈÁ¿£¬¿É̉Ô¼ÆËă³ö1mol¼×Í飨16g£©ÍêȫȼÉƠÉú³ÉÎȶ¨µÄÑơ»¯Îïʱ·Å³ö8Q kJµÄÈÈÁ¿£¬Ëù̉Ô¼×ÍéȼÉƠÈȵÄÈÈ»¯Ñ§·½³̀ʽΪCH4(g)+2O2(g)=CO2(g)+2H2O(l)¡¡¡¡¡÷H=-8QkJ/mol ¡£
£¨2£©̉ÑÖª¢ÙC(s)£«H2O(g)=CO(g)£«H2(g) ¦¤H£½£«130 kJ¡¤mol£1£¬¢Ú2C(s)£«O2(g)=2CO(g) ¦¤H£½£220 kJ¡¤mol£1£¬ÓÉ¢Ú-¢Ù![]() 2µĂ£¬2H2(g)£«O2(g)= 2H2O(g)£¬¦¤H£½£220 kJ¡¤mol£1 -£¨£«130 kJ¡¤mol£1£©
2µĂ£¬2H2(g)£«O2(g)= 2H2O(g)£¬¦¤H£½£220 kJ¡¤mol£1 -£¨£«130 kJ¡¤mol£1£©![]() 2=-480 kJ¡¤mol£1¡£¶Ï¿ª1 mol H¡ªH¼ü¡¢O===O¼ü·Ö±đĐè̉ªÎüÊƠ436 kJ¡¢496 kJµÄÈÈÁ¿£¬Éè¶Ï¿ª1 mol O¡ªH¼üĐè̉ªÎüÊƠµÄÈÈÁ¿Îªx£¬Ộ2
2=-480 kJ¡¤mol£1¡£¶Ï¿ª1 mol H¡ªH¼ü¡¢O===O¼ü·Ö±đĐè̉ªÎüÊƠ436 kJ¡¢496 kJµÄÈÈÁ¿£¬Éè¶Ï¿ª1 mol O¡ªH¼üĐè̉ªÎüÊƠµÄÈÈÁ¿Îªx£¬Ộ2![]() £¬½âÖ®µĂ£¬x=462. Ộ¶Ï¿ª1 mol O¡ªH¼üĐè̉ªÎüÊƠµÄÈÈÁ¿Îª462 kJ £¬Ñ¡B¡£
£¬½âÖ®µĂ£¬x=462. Ộ¶Ï¿ª1 mol O¡ªH¼üĐè̉ªÎüÊƠµÄÈÈÁ¿Îª462 kJ £¬Ñ¡B¡£
£¨3£©̉ÔCO2ºÍH2OΪÔÁÏÖƱ¸HCOOHºÍO2µÄÔµç³Ø×Ü·´Ó¦Îª2CO2+2H2O=2HCOOH+O2£¬ÓÉͼ¿ÉÖª£¬µç¼«aÉÏË®±äΪÑơÆø£¬·¢ÉúÁËÑơ»¯·´Ó¦£¬Ëù̉Ե缫aΪ¸º¼«£¬µç¼«·´Ó¦Ê½Îª2H2O+4e- = 4H++O2¡£µç¼«bΪƠư¼«£¬bµç¼«ÉÏ·¢Éú»¹Ô·´Ó¦£¬¶₫Ñơ»¯̀¼±»»¹ÔΪ¼×Ëᣬµç¼«b·¢ÉúµÄµç¼«·´Ó¦Ê½ÊÇ2CO2+4e-+4H+=2HCOOH¡£

 ÇáËÉ¿Î̀õ¥Ôª²âÊÔAB¾íϵÁĐ´đ°¸
ÇáËÉ¿Î̀õ¥Ôª²âÊÔAB¾íϵÁĐ´đ°¸ Đ¡̀â¿ñ×öϵÁĐ´đ°¸
Đ¡̀â¿ñ×öϵÁĐ´đ°¸¡¾̀âÄ¿¡¿ÏÂÁĐÄÚÈƯÓë½áÂÛ²»¶ÔÓ¦µÄÊÇ
Ñ¡Ïî | ÄÚÈƯ | ½áÂÛ |
A | H2O(g)±ä³ÉH2O(l) | ¸Ă¹ư³̀µÄ¦¤S£¼0 |
B | ÏơËáï§ÈÜÓÚË®¿É×Ô·¢½øĐĐ | ̣̉Ϊ¦¤S£¾0 |
C | ̉»¸ö·´Ó¦µÄ¦¤H£¾0¡¢¦¤S£¾0 | ¸Ă·´Ó¦̉»¶¨²»ÄÜ×Ô·¢½øĐĐ |
D | H2(g)£«F2(g)=2HF(g) ¦¤H£½£271 kJ¡¤mol£1¡¢ ¦¤S£½8 J¡¤mol£1¡¤K£1 | ¸Ă·´Ó¦ÔÚÈκÎζÈϾù¿É×Ô·¢½øĐĐ |
A.AB.BC.CD.D
¡¾̀âÄ¿¡¿Ï±íÊÇÑơ×岿·ÖÔªËصÄÏà¹ØĐÔÖÊ¡£
ÔªËØ ĐÔÖÊ | 8O | 16S | 34Se | 52Te |
Ö÷̉ª»¯ºÏ¼Û | -2 | -2¡¢+4¡¢+6 | -2¡¢+4¡¢+6 | |
Ô×Ӱ뾶 | Öđ½¥Ôö´ó | |||
µ¥ÖÊÓëH2 ·´Ó¦Çé¿ö | µăȼʱ ̉×»¯ºÏ | ¼ÓÈÈ »¯ºÏ | ¼ÓÈÈ ÄÑ»¯ºÏ | ²»ÄÜ Ö±½Ó»¯ºÏ |
Çë»Ø´đÏÂÁĐÎỀâ:
£¨1£©ÎøÔÚÖÜÆÚ±íÖĐλÖĂ____¡£
£¨2£©íڵĻ¯ºÏ¼Û¿ÉÄÜÓĐ____¡£
£¨3£©Ạ́¡¢Îø¡¢íÚµÄÇ⻯ÎïË®ÈÜ̉ºµÄËáĐÔÓÉÇ¿ÖÁÈơµÄ˳Đ̣ÊÇ__________(̀ѧʽ)¡£
£¨4£©ÇâÎøËáÓĐ½ÏÇ¿µÄ____(̀î¡°Ñơ»¯ĐÔ¡±»̣¡°»¹ÔĐÔ¡±),̣̉´Ë·ÅÔÚ¿ƠÆøÖĐ̉×±äÖÊ,Æä¿ÉÄÜ·¢ÉúµÄ»¯Ñ§·½³̀ʽΪ_____________¡£
£¨5£©Ñơ×åÔªËص¥ÖÊÓëH2·´Ó¦¹ư³̀ÖеÄ́ʱäÈçͼËùʾ,ÆäÖĐa¡¢b¡¢c¡¢d·Ö±đ±íʾÑơ×åÖĐij̉»ÔªËصĵ¥ÖÊ¡£Ộb´ú±í___,d´ú±í___(¾ùĐ´µ¥ÖÊĂû³Æ)¡£
