��Ŀ����
��������������ͭ�Ľᾧˮ����仯ѧʽΪCuSO4?5H2O���ڼ�������£����¶Ȳ�ͬ���������������һϵ�еı仯���õ���ͬ��ɵĹ��塣
��1����ȡ0.1000 g�������ʵĵ�����������ƿ�У�����0.1000 mol/L����������Һ28.00 mL����Ӧ��ȫ����������������0.1000 mol/L����ζ����յ㣬��������10.08 mL���������е�������������Ϊ___________��
����֪��CuSO4 + 2NaOH �� Cu(OH)2 + Na2SO4�������е����ʲ����ᡢ�Ӧ��
��2����1.250 g�����ĵ����������������м���һ��ʱ�䣬���ʣ���������Ϊ0.960 g��ʣ������нᾧˮ����������Ϊ__________(������λС��)��
��3������ˮ����ͭ������650�����ϣ��ɵõ���ɫ������ͭ����������������������Ļ�����塣�ֽ�9.600 g��ˮ����ͭ��ּ��ȷֽ�Ϊ����ͭ�������ɵ�����ͨ�����������ռ�����ʯ�ң������ռ�����4.416 g�������������ռ������������������ε����ʵ���֮�ȡ�
��4����ˮ����ͭ���ȷֽ������֮ͭǰ����һ�ֻ�ɫ�м����X���֣��仯ѧʽ���Ա�ʾΪCuaOb(SO4)c��a��b��cΪ����������X����ˮ�У��в��ܵ���ɫ����Y���ɣ���ѧʽΪCuSO4��nCu(OH)2����ͬʱ����2/3�����������ˮ������Y���м�����ˮ����ʧȥ11.9%����������֪X��Y��������ϡ���ᡣͨ������ȷ��X��Y�Ļ�ѧʽ��
��1����ȡ0.1000 g�������ʵĵ�����������ƿ�У�����0.1000 mol/L����������Һ28.00 mL����Ӧ��ȫ����������������0.1000 mol/L����ζ����յ㣬��������10.08 mL���������е�������������Ϊ___________��
����֪��CuSO4 + 2NaOH �� Cu(OH)2 + Na2SO4�������е����ʲ����ᡢ�Ӧ��
��2����1.250 g�����ĵ����������������м���һ��ʱ�䣬���ʣ���������Ϊ0.960 g��ʣ������нᾧˮ����������Ϊ__________(������λС��)��
��3������ˮ����ͭ������650�����ϣ��ɵõ���ɫ������ͭ����������������������Ļ�����塣�ֽ�9.600 g��ˮ����ͭ��ּ��ȷֽ�Ϊ����ͭ�������ɵ�����ͨ�����������ռ�����ʯ�ң������ռ�����4.416 g�������������ռ������������������ε����ʵ���֮�ȡ�
��4����ˮ����ͭ���ȷֽ������֮ͭǰ����һ�ֻ�ɫ�м����X���֣��仯ѧʽ���Ա�ʾΪCuaOb(SO4)c��a��b��cΪ����������X����ˮ�У��в��ܵ���ɫ����Y���ɣ���ѧʽΪCuSO4��nCu(OH)2����ͬʱ����2/3�����������ˮ������Y���м�����ˮ����ʧȥ11.9%����������֪X��Y��������ϡ���ᡣͨ������ȷ��X��Y�Ļ�ѧʽ��
��1��0.98��3�֣���
��2��0.167��3�֣���
��3��3:2��4�֣���
��4��X��Cu2OSO4��2�֣���Y��CuSO4��3Cu(OH)2��2�֣�
������̣�
�ȼ���Y�Ļ�ѧʽ��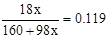 �����x=3
�����x=3
��ΪCuSO4��3Cu(OH)2
��2/3���������ˮ����Y������ϡ�����֪�ܽ�ijɷ�ΪCuSO4�������ʵ���Ϊ������2�����ɴ˿�֪��ɫ�м���ﺬ��Cu2+��SO42-�ĸ�����Ϊ2:1�����ݵ���غ��֪�仯ѧʽΪCu2OSO4��
��2��0.167��3�֣���
��3��3:2��4�֣���
��4��X��Cu2OSO4��2�֣���Y��CuSO4��3Cu(OH)2��2�֣�
������̣�
�ȼ���Y�Ļ�ѧʽ��
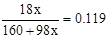 �����x=3
�����x=3��ΪCuSO4��3Cu(OH)2
��2/3���������ˮ����Y������ϡ�����֪�ܽ�ijɷ�ΪCuSO4�������ʵ���Ϊ������2�����ɴ˿�֪��ɫ�м���ﺬ��Cu2+��SO42-�ĸ�����Ϊ2:1�����ݵ���غ��֪�仯ѧʽΪCu2OSO4��
�����������1��n(NaOH)="0.1000" mol/L*0.028mL=2.8*10-3mol��n(H2SO4 )="0.1000" mol/L*0.0108 L=1.08*10-3mol���͵�����Ӧ�������������ʵ���Ϊ��2.8*10-3mol-2.16*10-3mol=6.4*10-4mol���������ʵ���Ϊ3.2*10-4mol������Ϊ3.2*10-4*250g��������������Ϊ3.2*10-4*250g/0.1g=0.8��
��2��1.250 g �����ĵ�������������ͭ������Ϊ1.25*160/250=0.8g��ʣ���������Ϊ0.960 g��ˮ������Ϊ0.16g��ʣ������нᾧˮ����������Ϊ0.16/0.96=0.167��
��3��9.600 g��ˮ����ͭ�����ʵ���Ϊ0.06mol�����ռ�����4.416 g���������ɵĶ���������������������֮�͡�����������������������ʵ����ֱ�Ϊx,y������x+y=0.06�� 64x+80y=4.416�����y=0.036,x=0.024���������ʵ���֮��Ϊ3��2��
��4���ȼ���Y�Ļ�ѧʽ��
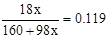 �����x=3 ��ΪCuSO4��3Cu(OH)2����2/3���������ˮ����Y������ϡ�����֪�ܽ�ijɷ�ΪCuSO4�������ʵ���Ϊ������2�����ɴ˿�֪��ɫ�м���ﺬ��Cu2+��SO42-�ĸ�����Ϊ2:1�����ݵ���غ��֪�仯ѧʽΪCu2OSO4��
�����x=3 ��ΪCuSO4��3Cu(OH)2����2/3���������ˮ����Y������ϡ�����֪�ܽ�ijɷ�ΪCuSO4�������ʵ���Ϊ������2�����ɴ˿�֪��ɫ�м���ﺬ��Cu2+��SO42-�ĸ�����Ϊ2:1�����ݵ���غ��֪�仯ѧʽΪCu2OSO4��
��ϰ��ϵ�д�
 ѧ���쳵�����ּ��������ҵ�½����������ϵ�д�
ѧ���쳵�����ּ��������ҵ�½����������ϵ�д� �����ѧСѧ�꼶�νӵ������㽭��ѧ������ϵ�д�
�����ѧСѧ�꼶�νӵ������㽭��ѧ������ϵ�д� Сѧ�����ҵ���ϴ�ѧ������ϵ�д�
Сѧ�����ҵ���ϴ�ѧ������ϵ�д� ���Ž�����ٰθ��νӹ㶫���������ϵ�д�
���Ž�����ٰθ��νӹ㶫���������ϵ�д�
�����Ŀ