��Ŀ����
 ����ͭ������ϡ���ᣬ������������Һ����ͭ���������Σ��ֽ�һ������ͭƬ���뵽100mLϡ������������Ļ����Һ�У�ͭƬ��ȫ�ܽ⣨�������ε�ˮ�⼰��Һ����ı仯����
����ͭ������ϡ���ᣬ������������Һ����ͭ���������Σ��ֽ�һ������ͭƬ���뵽100mLϡ������������Ļ����Һ�У�ͭƬ��ȫ�ܽ⣨�������ε�ˮ�⼰��Һ����ı仯������1��д��ͭ�ܽ������������Һ�����ӷ���ʽ
Cu+2Fe3+�TCu2++2Fe2+
Cu+2Fe3+�TCu2++2Fe2+
����2����ͭ��ȫ�ܽ�ʱ����Һ�е�Fe3+��Cu2+��H+�������ӵ����ʵ���Ũ����ȣ��Ҳ����Һ��pH=1�����ܽ�ͭ��������
0.64
0.64
g����Һ�е�c��SO2- 4 |
0.3
0.3
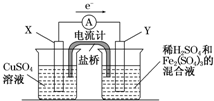
mol/L����3����������ͼ��ʾ��װ���з�����1���еķ�Ӧ����X����
����
����
���������������缫��ӦʽCu-2e-�TCu2+
Cu-2e-�TCu2+
��Y���IJ���������̼��ʯī���𡢲�������
̼��ʯī���𡢲�������
���缫��Ӧʽ2Fe3++2e-�T2Fe2+
2Fe3++2e-�T2Fe2+
����������1��ͭ�������ӷ�Ӧ�����������Ӻ�ͭ���ӣ�
��2������ԭ���غ����ͭ�����������ݵ���غ�������������Ũ�ȣ�
��3����������ͼ��ʾ��װ���з�����1���еķ�Ӧ����ͭ�缫�Ǹ���������ͭ���õĽ�����ķǽ�����������������ʧ���ӷ���������Ӧ�������ϵõ��ӷ�����ԭ��Ӧ��
��2������ԭ���غ����ͭ�����������ݵ���غ�������������Ũ�ȣ�
��3����������ͼ��ʾ��װ���з�����1���еķ�Ӧ����ͭ�缫�Ǹ���������ͭ���õĽ�����ķǽ�����������������ʧ���ӷ���������Ӧ�������ϵõ��ӷ�����ԭ��Ӧ��
����⣺��1��ͭ�������ӷ�Ӧ�����������Ӻ�ͭ���ӣ����ӷ�Ӧ����ʽΪ��Cu+2Fe3+�TCu2++2Fe2+���ʴ�Ϊ��Cu+2Fe3+�TCu2++2Fe2+��
��2����ͭ��ȫ�ܽ�ʱ����Һ�е�Fe3+��Cu2+��H+�������ӵ����ʵ���Ũ����ȣ��Ҳ����Һ��pH=1����c��H+��=c��Cu2+��=0.1mol/L������ԭ���غ��m��Cu��=CVM=0.1mol/L��0.1L��64g/mol=0.64g��
���ݵ���غ��2c��SO42-��=c��H+��+2c��Cu2+��+3c��Fe3+��=6c��H+��=0.6mol/L��c��SO42-��=0.3mol/L��
�ʴ�Ϊ��0.64��0.3mol/L��
��3����������ͼ��ʾ��װ���з�����1���еķ�Ӧ����ͭ�缫�Ǹ���������ͭ���õĽ�����ķǽ���������������X�缫�Ǹ�����
�����ϵ缫��ӦʽΪ��Cu-2e-�TCu2+��̼��ʯī���𡢲���������������
�����������ӵõ��ӷ�����ԭ��Ӧ���缫��ӦʽΪ��2Fe3++2e-�T2Fe2+��
�ʴ�Ϊ��������Cu-2e-�TCu2+��̼��ʯī���𡢲���������2Fe3++2e-�T2Fe2+��
��2����ͭ��ȫ�ܽ�ʱ����Һ�е�Fe3+��Cu2+��H+�������ӵ����ʵ���Ũ����ȣ��Ҳ����Һ��pH=1����c��H+��=c��Cu2+��=0.1mol/L������ԭ���غ��m��Cu��=CVM=0.1mol/L��0.1L��64g/mol=0.64g��
���ݵ���غ��2c��SO42-��=c��H+��+2c��Cu2+��+3c��Fe3+��=6c��H+��=0.6mol/L��c��SO42-��=0.3mol/L��
�ʴ�Ϊ��0.64��0.3mol/L��
��3����������ͼ��ʾ��װ���з�����1���еķ�Ӧ����ͭ�缫�Ǹ���������ͭ���õĽ�����ķǽ���������������X�缫�Ǹ�����
�����ϵ缫��ӦʽΪ��Cu-2e-�TCu2+��̼��ʯī���𡢲���������������
�����������ӵõ��ӷ�����ԭ��Ӧ���缫��ӦʽΪ��2Fe3++2e-�T2Fe2+��
�ʴ�Ϊ��������Cu-2e-�TCu2+��̼��ʯī���𡢲���������2Fe3++2e-�T2Fe2+��
���������⿼��ԭ���ԭ�������ʵ������йؼ����֪ʶ�㣬����ԭ��صĹ����������缫��Ӧ��ԭ���غ㡢����غ���������ɣ��ѶȲ���

��ϰ��ϵ�д�
�����Ŀ
 ��2010?����һģ����2011?�����ʼ죩����ͭ������ϡ���ᣬ������������Һ����ͭ���������Σ��ֽ�һ������ͭƬ���뵽100mLϡ������������Ļ��Һ�У�ͭƬ��ȫ�ܽ⣨�������ε�ˮ�⼰��Һ����ı仯��
��2010?����һģ����2011?�����ʼ죩����ͭ������ϡ���ᣬ������������Һ����ͭ���������Σ��ֽ�һ������ͭƬ���뵽100mLϡ������������Ļ��Һ�У�ͭƬ��ȫ�ܽ⣨�������ε�ˮ�⼰��Һ����ı仯��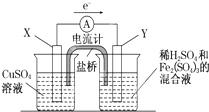 ����ͭ������ϡ���ᣬ������������Һ����ͭ���������Σ��ֽ�һ������ͭƬ���뵽100mLϡ������������Ļ����Һ�У�ͭƬ��ȫ�ܽ⣨�������ε�ˮ�⼰��Һ����ı仯����
����ͭ������ϡ���ᣬ������������Һ����ͭ���������Σ��ֽ�һ������ͭƬ���뵽100mLϡ������������Ļ����Һ�У�ͭƬ��ȫ�ܽ⣨�������ε�ˮ�⼰��Һ����ı仯����