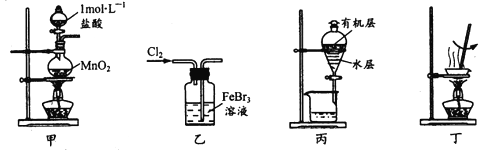
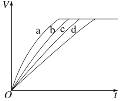
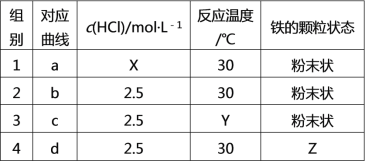��Ŀ����
����Ŀ������ʱ����H2CO3��Һ����μ���NaOH��Һ����Һ��H2CO3��HCO3-��CO32-�����ֲַ�����![]() (X)=
(X)=![]() ��pH�Ĺ�ϵ��ͼ��ʾ��
��pH�Ĺ�ϵ��ͼ��ʾ��

����˵����ȷ����
A. ��ӦHCO3-![]() H����CO32-��lgK=-6.4
H����CO32-��lgK=-6.4
B. pH�T8����Һ�У�c��Na����>c��HCO3-��
C. NaHCO3��Һ�е���������������Һ�����ԣ�c��Na�����Tc��Cl-��
D. ��pH=6.4����Һ�еμ�NaOH��Һ��pH=8����Ҫ���������ӷ�Ӧ��HCO3-��OH-�TCO32-��H2O
���𰸡�B
��������
A��pH�T6.4ʱ��c��HCO3-���Tc��H2CO3�����������ӦH2CO3![]() H++HCO3-��lgK��
H++HCO3-��lgK��
B�����ݵ���غ������
C�����ݵ���غ������
D��pH=8ʱ��̼��ת��Ϊ̼���������Ҫ���������ӷ�ӦΪH2CO3��OH-=HCO3-��H2O��
A��pH�T6.4ʱ��c��HCO3-���Tc��H2CO3������ӦH2CO3![]() H++HCO3-��lgK�Tlgc(H+)=lg10-6.4=һ6.4����A�����
H++HCO3-��lgK�Tlgc(H+)=lg10-6.4=һ6.4����A�����
B��pH�T8����Һ�У�c��H����<c��OH-�������ݵ���غ���c��Na+����c��H�����Tc��C1-��ʮc��HCO3-����2c��CO32-����c��OH-��������c��Na+��>c��C1-��ʮc��HCO3-����2c��CO32-������c��Na����>c��HCO3-��,��B��ȷ��
C����NaHCO3��Һ�е���������������Һ������ʱ��c��H�����Tc��OH-�������ݵ���غ���c��Na+����c��H�����Tc��C1-��ʮc��HCO3-����2c��CO32-����c��OH-������c��Na+���Tc��C1-��ʮc��HCO3-����2c��CO32-������C�����
D��pH�T6.4����Һ�к��е����ʵ�����NaHCO3��H2CO3����ͼ���֪��pH=8ʱ��Һ��HCO3-��������������H2CO3������Ҫ���������ӷ�ӦΪH2CO3��OH-=HCO3-��H2O����D�����
��ѡB��

����Ŀ��������KMnO4��H2C2O4����������Ӧ�о�Ӱ�췴Ӧ���ʵ����ء�һʵ��С����ͨ���ⶨ��λʱ��������CO2�����ʣ�̽��ij��Ӱ�컯ѧ��Ӧ���ʵ����أ����ʵ�鷽��������KMnO4��Һ���ữ����
ʵ����� | A��Һ | B��Һ |
�� | 20 mL 0.1 mol��L��1H2C2O4��Һ | 30 mL 0.01 mol��L��1KMnO4��Һ |
�� | 20 mL 0.2 mol��L��1H2C2O4��Һ | 30 mL 0.01 mol��L��1KMnO4��Һ |

��1���÷�Ӧ�����ӷ���ʽ ������֪H2C2O4�Ƕ�Ԫ������
��2����ʵ��̽������ ���ضԻ�ѧ��Ӧ���ʵ�Ӱ�졣��ͬʱ������Ͳ������CO2�������С��ϵ�� �� ����ʵ���������
��3����ʵ������2 minĩ�ռ���2.24 mL CO2����״������������2 minĩ�� c��MnO4������__________mol/L��������Һ���Ϊ50 mL��
��4����ͨ���ⶨһ��ʱ����CO2��������ȽϷ�Ӧ���ʣ���ʵ�黹��ͨ���ⶨ ���Ƚϻ�ѧ��Ӧ���ʡ���һ��������
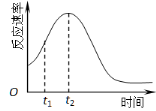 ��5��С��ͬѧ���ַ�Ӧ����������ͼ������t1��t2ʱ�������ʱ�����Ҫԭ������ǣ�
��5��С��ͬѧ���ַ�Ӧ����������ͼ������t1��t2ʱ�������ʱ�����Ҫԭ������ǣ�

�� ���� ��