��Ŀ����
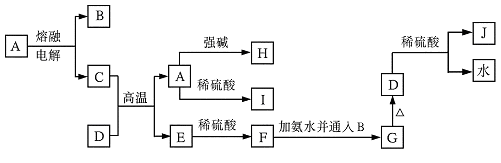
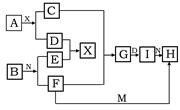
��10�֣�A��B��C��D�ǰ�ԭ��������С�������еĵڶ���������Ԫ�صĵ��ʡ�B��E��Ϊ��ɿ����ijɷ֡�F����ɫ��Ӧ�ʻ�ɫ����G�У��ǽ���Ԫ�������Ԫ�ص�ԭ�Ӹ�����Ϊ1��2����һ�������£�������֮����ת����ϵ����(ͼ�в��ֲ���δ�г�)��

����д���пհף�
(1)A ��C�� ��
(2)H�����ᷴӦ����E�Ļ�ѧ����ʽ�� ��
(3)E��F��Ӧ�Ļ�ѧ����ʽ�� ��
(4)F��G��ˮ��Һ��Ӧ����I��D�����ӷ���ʽ��

����д���пհף�
(1)A ��C�� ��
(2)H�����ᷴӦ����E�Ļ�ѧ����ʽ�� ��
(3)E��F��Ӧ�Ļ�ѧ����ʽ�� ��
(4)F��G��ˮ��Һ��Ӧ����I��D�����ӷ���ʽ��
(13��)
(1)A:̼(��C) C:��(��Na) (2)Na2CO3+2HCl=2NaCl+H2O+CO2��
(3)2CO2+2Na2O2=2Na2CO3+O2 ��4��Na2O2+S2-+2H2O=4OH-+S��+2Na+
(1)A:̼(��C) C:��(��Na) (2)Na2CO3+2HCl=2NaCl+H2O+CO2��
(3)2CO2+2Na2O2=2Na2CO3+O2 ��4��Na2O2+S2-+2H2O=4OH-+S��+2Na+
�ۺϿ��ǿ�֪B��E�ֱ�O2��CO2��������֪AΪC��F����ɫ��Ӧ�ʻ�ɫ�����Ƶ�CΪNa��FΪNa2O2����G�У��ǽ���Ԫ�������Ԫ���Ƶ�ԭ�Ӹ�����Ϊ1��2�����Ƶ�DԪ��Ϊ��
��4��Na2O2��Na2S��ˮ�з�Ӧ�������ʣ�����������ͬʱ��ֻ����NaOH���ʴ���
��4��Na2O2��Na2S��ˮ�з�Ӧ�������ʣ�����������ͬʱ��ֻ����NaOH���ʴ���

��ϰ��ϵ�д�
 ͬ����ϰǿ����չϵ�д�
ͬ����ϰǿ����չϵ�д�
�����Ŀ
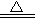 E+ F��+H2O
E+ F��+H2O