��Ŀ����
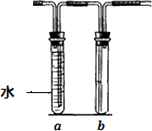
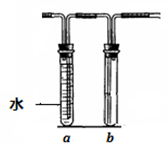
��16�֣���λͬѧ���ʵ��۲�ͭ��Ũ����ķ�Ӧ���������ɵ���������ʡ����������ͼ��ʾ��װ�ã��ڴ��Թ������2mLŨ���ᣬ�ô����ܺ�һ��С�Ľ����������ӿ��в���һ��ͭ˿�����ȣ��Ѳ�������������ͨ��Ʒ����Һ��ʯ����Һ�С�
��ش��������⣺

��1��д��ͭ��Ũ���ᷴӦ�Ļ�ѧ����ʽ�� ��
��2���Թ�b�е������� ��
ͨ���Թ�a��b������ó��Ľ����� ��
��3����ͬѧ��Ϊ��ͬѧ����Ʋ���ȫ������SO2�����ʣ������������Թܽ�������ʵ�顣����Ϊ���ӵ��Թ���ʢ�ŵ��Լ���A. ����ʯ��ˮ B. KMnO4��aq�� C. ������
���������ͬѧ����ѡ���ں�������д����ĸ���������ּ�˵��ʵ���Ŀ�ġ�
��4����ͬѧ���ۼ�ͬѧ��ʵ��װ�ü����ŵ�Ҳ�в��㣬�ŵ���
������֮���Ľ��ľ����ʩ�� ��
��5����������װ���д��Թܵ�Ũ���ỻ��ϡ���ᣬ�Թ�a��b�����¸ı䣨����aװ��ˮ��b���ã������ڴ��Թ��з����ķ�Ӧ�����ӷ���ʽΪ ��
�Թ�a�������� ��

��1��Cu + 2H2SO4��Ũ��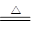 CuSO4 + SO2��+
2H2O ��2�֣�
CuSO4 + SO2��+
2H2O ��2�֣�
��2����ɫ��Һ���ɫ��1�֣���SO2��һ���������壬����Ư���ԣ���Ư��Ʒ�죩 ��2�֣�
��3��B����֤SO2�Ļ�ԭ�ԣ�C����֤SO2�������ԡ�(��1��)
��4��ʹ�ÿɳ鶯��ͭ˿����ʱ���Ʒ�Ӧ�Ŀ�ʼ�ͽ�������ԼҩƷ������������Ⱦ����IJ�����2�֣��� ��β��ͨ��NaOH��Һ�У�1�֣���
��5��3Cu2+ + 8H+ + 2NO3�� = 3Cu2+ + 2NO��+ 4H2O ��2�֣���
��ȥNO2����ˮ���ռ�������NO��2�֣���
����������1��Ũ�������ǿ�����ԣ��ڼ��ȵ�������������ͭ��������ͭ��
��2��Ũ����Ļ�ԭ������SO2��SO2����ˮ�����ԣ�������ɫ��Һ���ɫ��SO2��ʹƷ����Һ��ɫ������Ư���ԡ�
��3��SO2��S�Ļ��ϼ��ǣ�4�ۣ������м��̬�����������ԣ����л�ԭ�ԡ���˿���ͨ�����Ը��������Һ����֤�仹ԭ�ԡ�ͨ����������֤�������ԡ�
��4������װ�õ��ص���жϣ�ͨ��ʹ�ÿɳ鶯��ͭ˿����ʱ���Ʒ�Ӧ�Ŀ�ʼ�ͽ�������ԼҩƷ������������Ⱦ����IJ�����������SO2���ڴ�����ȾΪ����Ӧ��������Ҫ����β���������ɽ�β��ͨ������������Һ�С�
��5���������ǿ�����ԣ�Ҳ�ܰ�ͭ������������ͭ�������ᱻ��ԭ����NO2��NO���塣NO������ˮ��NO2����ˮ����NO�����Կ�������ˮ���ռ�������NO��

 �¿α�ͬ��ѵ��ϵ�д�
�¿α�ͬ��ѵ��ϵ�д� һ����ʦ����Ӧ����������һ��ȫϵ�д�
һ����ʦ����Ӧ����������һ��ȫϵ�д�