��Ŀ����
��16�֣�ֱ���ŷ�úȼ�ղ������������������صĻ������⣬������ͨ��װ��ʯ��ʯ��Һ������װ�ÿ��Գ�ȥ���еĶ�������������������ơ�����ƿ�����ͼ��ʾ��ѭ��ȼ��װ�õ�ȼ�Ϸ�Ӧ��������鷴Ӧ�������������ˮ��õ������������ʵĶ�����̼���Ӷ������ڶ�����̼�Ļ������ã��ﵽ����̼�ŷŵ�Ŀ�ġ�

��ش��������⣺
��1��úȼ�ղ���������ֱ���ŷŵ������У���������Ҫ���������� ������д��ĸ��ţ�
��2������������Ĺ����У����õ�ʯ��ʯ��Һ�ڽ�������װ��ǰ����ͨһ��ʱ�������̼������������Ч�ʣ�����ʱ���ƽ�Һ��pHֵ����ʱ��Һ���е���������ƿ��Ա���������������������ơ�
�ٶ�����̼��ʯ��ʯ��Һ��Ӧ�õ��IJ���Ϊ ��
����������Ʊ���������������������ƵĻ�ѧ����ʽΪ ��
��3����֪1mol CH4��ȼ�Ϸ�Ӧ������ȫ��Ӧ������̬ˮʱ���� 160.1kJ��1mol CH4����������ȫȼ��������̬ˮʱ����802.3kJ��д��������Ӧ���з������Ȼ�ѧ����ʽ�� ��
��4�����յ�CO2�뱽������һ��������Ӧ�����л���M���仯ѧʽΪC7H5O3Na��M��ϡ�����ữ��õ�һ��ҩ���м���N��N�Ľṹ��ʽΪ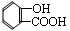 ��
��
��M�Ľṹ��ʽΪ ��
�ڷ������ޡ�O��O����ȩ���뱽��ֱ��������N��ͬ���칹�干�� �֡�

��ش��������⣺
��1��úȼ�ղ���������ֱ���ŷŵ������У���������Ҫ���������� ������д��ĸ��ţ�
| A������ЧӦ | B������ | C���۳���Ⱦ | D��ˮ�帻Ӫ���� |
�ٶ�����̼��ʯ��ʯ��Һ��Ӧ�õ��IJ���Ϊ ��
����������Ʊ���������������������ƵĻ�ѧ����ʽΪ ��
��3����֪1mol CH4��ȼ�Ϸ�Ӧ������ȫ��Ӧ������̬ˮʱ���� 160.1kJ��1mol CH4����������ȫȼ��������̬ˮʱ����802.3kJ��д��������Ӧ���з������Ȼ�ѧ����ʽ�� ��
��4�����յ�CO2�뱽������һ��������Ӧ�����л���M���仯ѧʽΪC7H5O3Na��M��ϡ�����ữ��õ�һ��ҩ���м���N��N�Ľṹ��ʽΪ
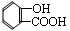 ��
����M�Ľṹ��ʽΪ ��
�ڷ������ޡ�O��O����ȩ���뱽��ֱ��������N��ͬ���칹�干�� �֡�
����1��ABC��3�֣���2����Ca(HCO3)2��̼����ƣ�2�֣���Ca(HSO3)2+O2=CaSO4��+ H2SO4��3�֣�
��3��CaS(s)+ 2O2(g)= CaSO4(s)����H="-962.4" KJ/mol����3�֣�
��4���� ��2�֣���6��3�֣�
��2�֣���6��3�֣�
��3��CaS(s)+ 2O2(g)= CaSO4(s)����H="-962.4" KJ/mol����3�֣�
��4����
 ��2�֣���6��3�֣�
��2�֣���6��3�֣�����1��úȼ�ղ�����������Ҫ��CO2��CO��SO2�ͷ۳���ֱ���ŷŵ������У�CO2��������ЧӦ��SO2�������꣬�۳�����۳���Ⱦ����ѡABC��
��2���ٶ�����̼��ʯ��ʯ��Һ��Ӧ�õ��IJ���Ϊ̼����ƣ�����������Ʊ���������������������ƵĻ�ѧ����ʽΪCa(HSO3)2+O2=CaSO4��+ H2SO4��
��3��CH4��ȼ�Ϸ�Ӧ���еķ�ӦΪCH4(g) +CaSO4(s)="CaS(s)+" CO2(g)+2H2O(g)����H1=+ 160.1Kj/mol
CH4������ȼ�շ�ӦΪCH4(g) +2O2(g) = CO2(g)+2H2O(g) ����H2="-802.3kJ" KJ/mol����ͼ�п��Կ�����������Ӧ���з����ķ�ӦΪ�Ȼ�ѧ����ʽCaS(s)+ 2O2(g)= CaSO4(s)�����ݸ�˹���ɣ������������Ȼ�ѧ����ʽ�ϲ��ɵ�CaS(s)+ 2O2(g)= CaSO4(s)����H=��H2-��H1="-962.4" KJ/mol��
��4�������ڷ��ǻ������������Ȼ�������N����֪��MΪ ����N��ͬ���칹���У�����ȩ���⣬Ӧ�û��������ǻ�����ȩ���뱽��ֱ��������N��ͬ���칹��������6�֣�
����N��ͬ���칹���У�����ȩ���⣬Ӧ�û��������ǻ�����ȩ���뱽��ֱ��������N��ͬ���칹��������6�֣�
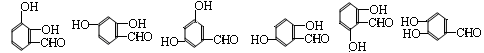
���㶨λ�����⿼�黯ѧ��ҵ���̣��漰���˻�ѧʽ���ƶϡ�����ʽ��д���Ȼ�ѧ����ʽ����д��ͬ���칹�����д��
��2���ٶ�����̼��ʯ��ʯ��Һ��Ӧ�õ��IJ���Ϊ̼����ƣ�����������Ʊ���������������������ƵĻ�ѧ����ʽΪCa(HSO3)2+O2=CaSO4��+ H2SO4��
��3��CH4��ȼ�Ϸ�Ӧ���еķ�ӦΪCH4(g) +CaSO4(s)="CaS(s)+" CO2(g)+2H2O(g)����H1=+ 160.1Kj/mol
CH4������ȼ�շ�ӦΪCH4(g) +2O2(g) = CO2(g)+2H2O(g) ����H2="-802.3kJ" KJ/mol����ͼ�п��Կ�����������Ӧ���з����ķ�ӦΪ�Ȼ�ѧ����ʽCaS(s)+ 2O2(g)= CaSO4(s)�����ݸ�˹���ɣ������������Ȼ�ѧ����ʽ�ϲ��ɵ�CaS(s)+ 2O2(g)= CaSO4(s)����H=��H2-��H1="-962.4" KJ/mol��
��4�������ڷ��ǻ������������Ȼ�������N����֪��MΪ
 ����N��ͬ���칹���У�����ȩ���⣬Ӧ�û��������ǻ�����ȩ���뱽��ֱ��������N��ͬ���칹��������6�֣�
����N��ͬ���칹���У�����ȩ���⣬Ӧ�û��������ǻ�����ȩ���뱽��ֱ��������N��ͬ���칹��������6�֣�
���㶨λ�����⿼�黯ѧ��ҵ���̣��漰���˻�ѧʽ���ƶϡ�����ʽ��д���Ȼ�ѧ����ʽ����д��ͬ���칹�����д��

��ϰ��ϵ�д�
 ��ѧ����ͬ����ϰϵ�д�
��ѧ����ͬ����ϰϵ�д� ��ǰ�κ�ͬ����ϰϵ�д�
��ǰ�κ�ͬ����ϰϵ�д� ����С��ҵϵ�д�
����С��ҵϵ�д�
�����Ŀ
