��Ŀ����
Ϊ�ⶨij��������Na2O��Na2O2��Ʒ�Ĵ��ȣ�3��С��ֱ�������·���������ȷ������Ʒmg��Ȼ�������·�������ʵ�飺
[����һ]������Ʒ��ˮ��ַ�Ӧ��ʹ������O2ͨ�����ȵ�ͭ�ۣ���÷�Ӧ����������ͭ������Ϊng��ͨ���������������Na2O2�ĺ������˷����ⶨ�Ľ�����ϴ���Ҫԭ���ǣ�______��
[������]������Ʒ�������̼��Ӧ��ͨ���ⶨ��Ӧ����������������������Ʒ��Na2O2�ĺ�����
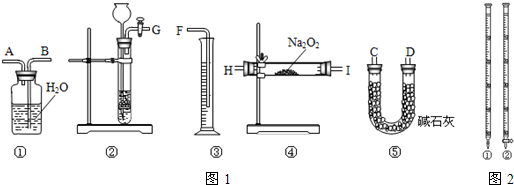
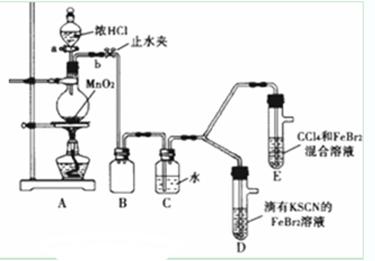
��1�����˷�����������ȡCO2��ʵ��ʹ�õ����������Ӵ�����______������д��ͼ1��ʾ������ţ�
��2��װ�âݵ������ǣ�______��
[������]���ⶨ��Ʒ��ˮ��ַ�Ӧ����Һ�����Vml���ٴ���ȡV1mL��Һ��װ����ƿ���ñ�Ũ�ȵ�������еζ���ȷ����Һ��Ũ�ȣ��ټ������Ʒ��Na2O2�ĺ�����
��1���˷��������ζ�ʱ��ѡ�õĵζ���Ϊ______������ͼ2��ʾ������ţ���
��2�����ü�����ָʾ�����ﵽ�ζ��յ�ʱ������Ϊ______������Ϊ���������������вⶨ����Ƚ�ȷ����______��
[����һ]������Ʒ��ˮ��ַ�Ӧ��ʹ������O2ͨ�����ȵ�ͭ�ۣ���÷�Ӧ����������ͭ������Ϊng��ͨ���������������Na2O2�ĺ������˷����ⶨ�Ľ�����ϴ���Ҫԭ���ǣ�______��
[������]������Ʒ�������̼��Ӧ��ͨ���ⶨ��Ӧ����������������������Ʒ��Na2O2�ĺ�����

��1�����˷�����������ȡCO2��ʵ��ʹ�õ����������Ӵ�����______������д��ͼ1��ʾ������ţ�
��2��װ�âݵ������ǣ�______��
[������]���ⶨ��Ʒ��ˮ��ַ�Ӧ����Һ�����Vml���ٴ���ȡV1mL��Һ��װ����ƿ���ñ�Ũ�ȵ�������еζ���ȷ����Һ��Ũ�ȣ��ټ������Ʒ��Na2O2�ĺ�����
��1���˷��������ζ�ʱ��ѡ�õĵζ���Ϊ______������ͼ2��ʾ������ţ���
��2�����ü�����ָʾ�����ﵽ�ζ��յ�ʱ������Ϊ______������Ϊ���������������вⶨ����Ƚ�ȷ����______��
����һ������Ʒ��ˮ��ַ�Ӧ��ʹ������O2ͨ�����ȵ�ͭ�ۣ���÷�Ӧ����������ͭ������Ϊng��ͨ���������������Na2O2�ĺ�����������ͭ�۲�һ��ȫ���������
����ͭ�����Ի������
�ʴ�Ϊ��O2��Cu��Ӧʱ����ȫ��ת����CuO��
��������
��1������Ʒ�������̼��Ӧ��ͨ���ⶨ��Ӧ����������������������Ʒ��Na2O2�ĺ�����ʵ��װ�����Ϊ������װ�â��Ʊ�������̼��ͨ��װ�âܺ���Ʒ�й������Ʒ�Ӧ��ͨ��װ�âݳ�ȥ�����Ķ�����̼�����â٢۲�ȡ��ˮ���������ⶨ�������������������Ӧ�̽�����������������˳��Ϊ���ڢܢݢ٢ۣ�
�ʴ�Ϊ�����������Ӵ����Ǣۢܢݢ٢ۣ�
��2��װ�â������ü�ʯ�ҳ�ȥ�����й����Ķ�����̼���壬����Ӱ������������ⶨ��
�ʴ�Ϊ����ȥO2�л��е�CO2���壻
��������
��1���˵ζ�ʵ������������ζ�����������Һ������Һ����ʢ����ʽ�ζ����н��еζ�ʵ�飬�ʴ�Ϊ���ڣ�
��2���������ζ�ָʾ�����ζ��յ�����Һ�л�ɫ�仯Ϊ��ɫ�Ұ���Ӳ��仯������һ��ͭ��һ��ȫ���仯Ϊ����ͭ���������У�װ���е����岻һ��ȫ���ϳ����������Ǿ�ȷ�ⶨ������Һ��Ũ�Ƚ����ȷ��
�ʴ�Ϊ����Һ��ɫ�ɻƱ���Ұ���Ӳ���ɫ����ȷ���Ƿ�������
����ͭ�����Ի������
�ʴ�Ϊ��O2��Cu��Ӧʱ����ȫ��ת����CuO��
��������
��1������Ʒ�������̼��Ӧ��ͨ���ⶨ��Ӧ����������������������Ʒ��Na2O2�ĺ�����ʵ��װ�����Ϊ������װ�â��Ʊ�������̼��ͨ��װ�âܺ���Ʒ�й������Ʒ�Ӧ��ͨ��װ�âݳ�ȥ�����Ķ�����̼�����â٢۲�ȡ��ˮ���������ⶨ�������������������Ӧ�̽�����������������˳��Ϊ���ڢܢݢ٢ۣ�
�ʴ�Ϊ�����������Ӵ����Ǣۢܢݢ٢ۣ�
��2��װ�â������ü�ʯ�ҳ�ȥ�����й����Ķ�����̼���壬����Ӱ������������ⶨ��
�ʴ�Ϊ����ȥO2�л��е�CO2���壻
��������
��1���˵ζ�ʵ������������ζ�����������Һ������Һ����ʢ����ʽ�ζ����н��еζ�ʵ�飬�ʴ�Ϊ���ڣ�
��2���������ζ�ָʾ�����ζ��յ�����Һ�л�ɫ�仯Ϊ��ɫ�Ұ���Ӳ��仯������һ��ͭ��һ��ȫ���仯Ϊ����ͭ���������У�װ���е����岻һ��ȫ���ϳ����������Ǿ�ȷ�ⶨ������Һ��Ũ�Ƚ����ȷ��
�ʴ�Ϊ����Һ��ɫ�ɻƱ���Ұ���Ӳ���ɫ����ȷ���Ƿ�������

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ