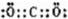��Ŀ����
��14�֣��±������ڱ��е�һ���֣�����a��n�����ڱ��е�λ�ã���Ҫ��ش����⣺
| | ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0 |
| 1 | a | | | |||||
| 2 | | | | b | c | | d | e |
| 3 | f | g | h | i | j | k | m | n |
(2)����������ˮ�����м�����ǿ���� �������Ե��� ��д�������������Ʒ�Ӧ�����ӷ���ʽ ������������������С����û�ѧʽ��ʾ��
(3)��b��c��d��f��g��h�У�ԭ�Ӱ뾶�Ӵ�С��˳��Ϊ���� ������Ԫ�ط��ű�ʾ����
(4) �Ƚ�k��m����̬�⻯����ȶ��� �����û�ѧʽ��ʾ��
(1)��������(2)Na��AL�� AL(OH)3 + OH-=Al3+ + 2H2O(3)Na>Mg>Al>C>N (4)H2S<HCl
AL(OH)3 + OH-=Al3+ + 2H2O(3)Na>Mg>Al>C>N (4)H2S<HCl
����

��ϰ��ϵ�д�
�����Ŀ
�±�ΪԪ�����ڱ���һ���������Ԫ�آ�-���ڱ��е�λ�ã��û�ѧ����ش��������⣺
��1����д���ڵ�Ԫ�ط���
��2����д���۵����������ĵ���ʽ ��
��3���ȽϢݡ��ޡ����ԭ�Ӱ뾶�ɴ�С��˳��Ϊ����Ԫ�ط��ű�ʾ��
��4���ȽϢۡ��ܡ������ۺ������������ǿ������˳���ǣ�����Ļ�ѧʽ��ʾ�� ��
��5��д����Ԫ�آ�-��������������Ӧˮ�������ǿ������ǿ������֮��Ļ�ѧ��Ӧ����ʽ
��6���ߢ���Ԫ����Ƚϣ������Խ�ǿ���� �������ƣ���������֤�ý��۵�ʵ���� �����ţ���
��a�����ڿ����з����Ѿõ�������Ԫ�صĿ�״���ʷֱ������ˮ��
��b������״����С��ͬ��������Ԫ�صĵ��ʷֱ��ͬŨ�ȵ����ᷴӦ
��c������״����С��ͬ������Ԫ�صĵ��ʷֱ����ˮ���ã��������̪��Һ
��d���Ƚ�������Ԫ�ص���̬�⻯����ȶ��ԣ�
| ������ | IA | |||||||
| 1 | �� | ��A | ��A | ��A | ��A | ��A | ��A | |
| 2 | �� | �� | �� | �� | ||||
| 3 | �� | �� | �� | �� | �� | |||
��2����д���۵����������ĵ���ʽ ��
��3���ȽϢݡ��ޡ����ԭ�Ӱ뾶�ɴ�С��˳��Ϊ����Ԫ�ط��ű�ʾ��
��4���ȽϢۡ��ܡ������ۺ������������ǿ������˳���ǣ�����Ļ�ѧʽ��ʾ�� ��
��5��д����Ԫ�آ�-��������������Ӧˮ�������ǿ������ǿ������֮��Ļ�ѧ��Ӧ����ʽ
��6���ߢ���Ԫ����Ƚϣ������Խ�ǿ���� �������ƣ���������֤�ý��۵�ʵ���� �����ţ���
��a�����ڿ����з����Ѿõ�������Ԫ�صĿ�״���ʷֱ������ˮ��
��b������״����С��ͬ��������Ԫ�صĵ��ʷֱ��ͬŨ�ȵ����ᷴӦ
��c������״����С��ͬ������Ԫ�صĵ��ʷֱ����ˮ���ã��������̪��Һ
��d���Ƚ�������Ԫ�ص���̬�⻯����ȶ��ԣ�