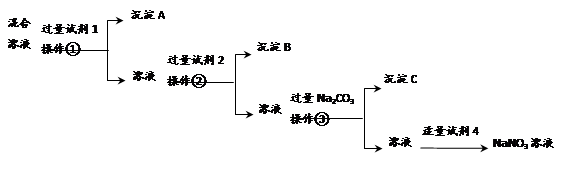��Ŀ����
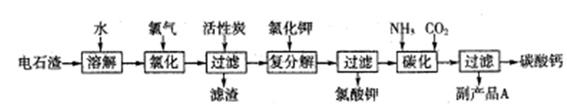
���Ե�ʯ��(��Ҫ�ɷ���Ca(OH)2����SiO2�Լ�������������)Ϊԭ�������������������̼��Ƶ��������£�
�ش��������⣺
��1����ʯ������ˮ�γɵ�ʯ����ʱ��������Ҫ��Ӧ�Ļ�ѧ����ʽΪ��
��2���Ȼ����̵��¶ȿ�����75��80�棬�ù�����Ҫ��Ӧ�����ӷ���ʽΪ��
��3���������м������̿��������
��4��̼�������У�������Һ��ͨ�백������ͨ��CO2��
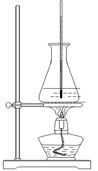
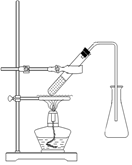
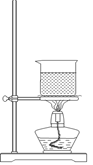
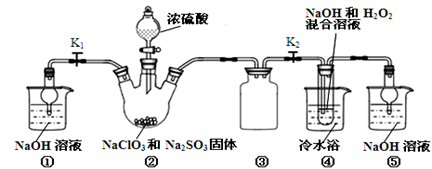
��ʵ����ͨ�����ü����Ȼ�狀��������ƻ����ķ�����ȡ������ijѧϰС��ѡȡ��ͼ��������װ����ȡ���ռ������İ�����

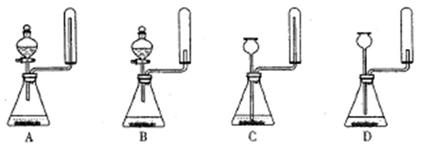
����������������Ӹ������ӿڣ�����Ϊ��ȷ��˳��Ϊa�� �� �� �� ��i��������i����©���������� ��
��ʵ�����л����ù����������ƺ�Ũ��ˮ��ȡ�����������������ʺ���ɸ�ʵ��ļ���װ���� (����)
��5������ƷA�Ļ�ѧʽΪ ��

�ش��������⣺
��1����ʯ������ˮ�γɵ�ʯ����ʱ��������Ҫ��Ӧ�Ļ�ѧ����ʽΪ��
��2���Ȼ����̵��¶ȿ�����75��80�棬�ù�����Ҫ��Ӧ�����ӷ���ʽΪ��
��3���������м������̿��������
��4��̼�������У�������Һ��ͨ�백������ͨ��CO2��
��ʵ����ͨ�����ü����Ȼ�狀��������ƻ����ķ�����ȡ������ijѧϰС��ѡȡ��ͼ��������װ����ȡ���ռ������İ�����

����������������Ӹ������ӿڣ�����Ϊ��ȷ��˳��Ϊa�� �� �� �� ��i��������i����©���������� ��
��ʵ�����л����ù����������ƺ�Ũ��ˮ��ȡ�����������������ʺ���ɸ�ʵ��ļ���װ���� (����)

��5������ƷA�Ļ�ѧʽΪ ��
��1��Ca(OH)2+SiO2=CaSiO3+H2O;��2��3Cl2+6OH-=ClO3-+5Cl-+3H2O��3��������������ֹ�ں������������������Ⱦ��������4����a��g,h��e,d��i;��ֹ���� ��A ��NH4Cl
����������������Һ��ͨ�����������������·����ķ�ӦΪ3Cl2+6OH-=ClO3-+5Cl-+3H2O�������ж��������գ����ü����Ȼ�狀��������ƻ����ķ�����ȡ�������������ü�ʯ�ң��ռ�ʱ����̹ܽ����������������ա��ҵ���©����ֹ������

��ϰ��ϵ�д�
�����Ŀ


 Mg(ClO3)2��2NaCl����
Mg(ClO3)2��2NaCl����
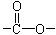 ������˴Ź�������ͼ��ʾ�����ط壬�����֮��Ϊ3��2��3�����л��ﲻ����CH3��O������A�Ľṹ��ʽΪ
������˴Ź�������ͼ��ʾ�����ط壬�����֮��Ϊ3��2��3�����л��ﲻ����CH3��O������A�Ľṹ��ʽΪ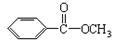 )�dz����㾫���㷺����ʳƷ����ױƷ����ҵ���ɴ���Ȼ������ȡ��Ҳ���˹��ϳɡ�ʵ��������ʳƷ������[��Ҫ�ɷ�Ϊ��������(
)�dz����㾫���㷺����ʳƷ����ױƷ����ҵ���ɴ���Ȼ������ȡ��Ҳ���˹��ϳɡ�ʵ��������ʳƷ������[��Ҫ�ɷ�Ϊ��������(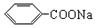 )]���״�Ϊԭ���Ʊ��������������֪��
)]���״�Ϊԭ���Ʊ��������������֪��