��Ŀ����
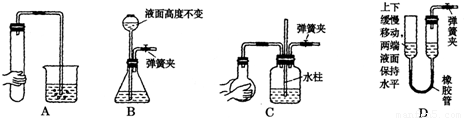
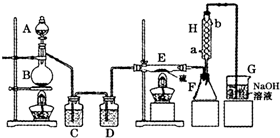
��ʵ������������װ�ý����й�ʵ��̽������ش��������⣺�������Ʊ�ʱ����װ�õ������ԣ�����ͼ��ʾ��װ�ã��϶������������Լ��Ҫ�����______��
����������װ�â���ȡ���ռ������NH3����ƿ��װ���Լ���______���ռ�װ��Ӧѡ��
______����װ����ţ���֤���������ռ����IJ�����______��
���Ȼ�����S2Cl2���ڹ�ҵ����������Ϊ��ʵ���Һϳ�S2Cl2��ij��ѧ�о���ѧϰС��������й����ϣ��õ�������Ϣ��
�ٽ������������110�桫140������Ӧ�����ɵ�S2Cl2��Ʒ��
���й����ʵIJ����������±���
| ���� | �۵�/�� | �е�� | ��ѧ���� |
| S | 112.8 | 444.6 | �� |
| S2Cl2 | -77 | 137 | ��ˮ����HCl��SO2��S��300��������ȫ�ֽ⣻ S2Cl2+Cl2  2SCl2 2SCl2 |
��1��B�з�Ӧ�����ӷ���ʽ��______ Mn2++Cl2��+2H2O
���𰸡������������ܷ��γ��ȶ���Һ����жϣ�
���������ƺ�ˮ��Ӧ���ɼ�Ҹ÷�Ӧ���ȣ���ˮ�ܵ�������������ӣ�Ҳ�ֽܷ�Ϊ������ˮ�����߽�Ϸ�����
���ݰ������ܶȡ������ж��ռ������������Ȼ���Ͱ����������Ȼ�粒�������ְ��̣�������ʹʪ��ĺ�ɫʯ����ֽ�������ݴ˷�����
��1��B�з�����Ӧ�����ӷ���ʽ��Ũ���������������ȡ������
��2����������ʱ����ˮ�½��ϳ���
��3������Ϣ��֪�������¶ȡ�ˮ������������HCl��S��SO2��SCl2���ʣ�
��4������Ϣ��֪S2Cl2��ˮˮ�⣬H������������Һ��ˮ�����ӷ�����G�У�
����⣺��A��B��C�����γɵ��ȶ���Һ�������ж�װ�õ��������Ƿ���ã�Dװ����Һ�汣��ˮƽʱ��������ѹ���ұ�װ���е�ѹǿ��ȣ�û��ѹǿ�����Բ��ܼ���װ�õ������ԣ��ʴ�Ϊ��D��
��NH3?H2O?NH4++OH-��NH3?H2O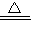 NH3��+H2O��CaO+H2O=Ca��OH��2����ʯ�Һ�ˮ��Ӧ���ɼ�ͬʱ�ų������ȣ��������ڰ����ݳ���
NH3��+H2O��CaO+H2O=Ca��OH��2����ʯ�Һ�ˮ��Ӧ���ɼ�ͬʱ�ų������ȣ��������ڰ����ݳ���
�ʴ�Ϊ����ʯ�ң�
������������ˮ�����Բ�������ˮ���ռ����������ܶ�С�ڿ������ܶȣ����Ա����������ſ������ռ�����ѡ�ޣ�
�ʴ�Ϊ���ޣ�
�ò�����պȡ����Ũ������ռ�NH3���Թܿڣ����������̣�˵���Թ����ռ���NH3����֮����û���ռ�������ʪ��ĺ�ɫʯ����ֽ�����ռ�NH3���Թܿڣ���ʪ��ĺ�ɫʯ����ֽ��������˵��NH3���ռ�������֮����û���ռ�����
�ʴ�Ϊ����ʪ��ĺ�ɫʯ����ֽ����ƿ�ڣ���ֽ��������պ��Ũ����IJ���������ƿ�ڣ��а�������
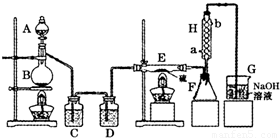
��1���ù�Һ���ȵķ�����Cl2������Ũ����Ͷ������̷�Ӧ��ȡ����Ӧ���ӷ���ʽΪ��MnO2+4H++2Cl-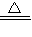 Mn2++Cl2��+2H2O���ʴ�Ϊ��MnO2+4H++2Cl-
Mn2++Cl2��+2H2O���ʴ�Ϊ��MnO2+4H++2Cl- 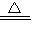 Mn2++Cl2��+2H2O��
Mn2++Cl2��+2H2O��
��2������ʱ����ˮ�½��ϳ����ʴ�Ϊ��a��b��
��3������Ϣ��֪���������¶ȡ�ˮ������������HCl��S��SO2��SCl2���ʣ����μ�������죬����ˮ����û�б��������գ�����E���У���Ӧ����Ũ����ĵ��ٲ�Ҫ���죮�ʴ�Ϊ������Ũ����ĵ��ٲ�Ҫ���죻
��4��G�ռ���Ʒ��H������������Һ��ˮ�����ӷ�������G�У���ʹS2Cl2ˮ�⣮��G��H֮�����Ӹ���װ�ã�
�ʴ�Ϊ����G��H֮�����Ӹ���װ�ã�G�мӷ�����װ�ã�
������������Ҫ����ѧ����ʵ��ԭ����װ�õ����⡢���ۣ��Լ��������������Ϣ��ƺ�����ʵ�����̺�ʵ�����������ȣ�������ѧ������֪ʶ���������������������
���������ƺ�ˮ��Ӧ���ɼ�Ҹ÷�Ӧ���ȣ���ˮ�ܵ�������������ӣ�Ҳ�ֽܷ�Ϊ������ˮ�����߽�Ϸ�����
���ݰ������ܶȡ������ж��ռ������������Ȼ���Ͱ����������Ȼ�粒�������ְ��̣�������ʹʪ��ĺ�ɫʯ����ֽ�������ݴ˷�����
��1��B�з�����Ӧ�����ӷ���ʽ��Ũ���������������ȡ������
��2����������ʱ����ˮ�½��ϳ���
��3������Ϣ��֪�������¶ȡ�ˮ������������HCl��S��SO2��SCl2���ʣ�
��4������Ϣ��֪S2Cl2��ˮˮ�⣬H������������Һ��ˮ�����ӷ�����G�У�
����⣺��A��B��C�����γɵ��ȶ���Һ�������ж�װ�õ��������Ƿ���ã�Dװ����Һ�汣��ˮƽʱ��������ѹ���ұ�װ���е�ѹǿ��ȣ�û��ѹǿ�����Բ��ܼ���װ�õ������ԣ��ʴ�Ϊ��D��
��NH3?H2O?NH4++OH-��NH3?H2O
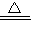 NH3��+H2O��CaO+H2O=Ca��OH��2����ʯ�Һ�ˮ��Ӧ���ɼ�ͬʱ�ų������ȣ��������ڰ����ݳ���
NH3��+H2O��CaO+H2O=Ca��OH��2����ʯ�Һ�ˮ��Ӧ���ɼ�ͬʱ�ų������ȣ��������ڰ����ݳ����ʴ�Ϊ����ʯ�ң�
������������ˮ�����Բ�������ˮ���ռ����������ܶ�С�ڿ������ܶȣ����Ա����������ſ������ռ�����ѡ�ޣ�
�ʴ�Ϊ���ޣ�
�ò�����պȡ����Ũ������ռ�NH3���Թܿڣ����������̣�˵���Թ����ռ���NH3����֮����û���ռ�������ʪ��ĺ�ɫʯ����ֽ�����ռ�NH3���Թܿڣ���ʪ��ĺ�ɫʯ����ֽ��������˵��NH3���ռ�������֮����û���ռ�����
�ʴ�Ϊ����ʪ��ĺ�ɫʯ����ֽ����ƿ�ڣ���ֽ��������պ��Ũ����IJ���������ƿ�ڣ��а�������
��1���ù�Һ���ȵķ�����Cl2������Ũ����Ͷ������̷�Ӧ��ȡ����Ӧ���ӷ���ʽΪ��MnO2+4H++2Cl-
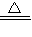 Mn2++Cl2��+2H2O���ʴ�Ϊ��MnO2+4H++2Cl-
Mn2++Cl2��+2H2O���ʴ�Ϊ��MnO2+4H++2Cl-  Mn2++Cl2��+2H2O��
Mn2++Cl2��+2H2O����2������ʱ����ˮ�½��ϳ����ʴ�Ϊ��a��b��
��3������Ϣ��֪���������¶ȡ�ˮ������������HCl��S��SO2��SCl2���ʣ����μ�������죬����ˮ����û�б��������գ�����E���У���Ӧ����Ũ����ĵ��ٲ�Ҫ���죮�ʴ�Ϊ������Ũ����ĵ��ٲ�Ҫ���죻
��4��G�ռ���Ʒ��H������������Һ��ˮ�����ӷ�������G�У���ʹS2Cl2ˮ�⣮��G��H֮�����Ӹ���װ�ã�
�ʴ�Ϊ����G��H֮�����Ӹ���װ�ã�G�мӷ�����װ�ã�
������������Ҫ����ѧ����ʵ��ԭ����װ�õ����⡢���ۣ��Լ��������������Ϣ��ƺ�����ʵ�����̺�ʵ�����������ȣ�������ѧ������֪ʶ���������������������

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
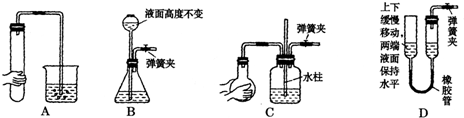










 ��NaOH��Һ480mL�����Ƹ���Һ��������������������ƽ�����룩����ͷ�ιܡ�ҩ�ס�����������ȱ�ٵ������� ��
��NaOH��Һ480mL�����Ƹ���Һ��������������������ƽ�����룩����ͷ�ιܡ�ҩ�ס�����������ȱ�ٵ������� ��
