��Ŀ����
����0.175mol/L��������Һ500mL(��֪����ĵ��볣��Ka=1.75x10 )
)
��1��д��������ˮ�ⷴӦ�Ļ�ѧ����ʽ_____________________��
��2������ͼ����˵�������Ƶ�ˮ�ⷴӦ�ﵽƽ�����_____________________��
��3���ڴ�������Һ�м��������������ʣ�ˮ��ƽ��������Ӧ�����ƶ�����
A�������� B��������� C������ƹ��� D���Ȼ�粒���
��4���ڴ�������Һ�м����������������Һ����Ũ�ȵĹ�ϵʽ�ܳ�������
A��c(CH3COO-)+c(CH3COOH)��c(Na+)
B��c(Na+)+c(CH3COO-)��c(H+)��c(OH-)
C��c(CH3COO-)��c(Na+)��c(H+)��c(OH-)
D��c(CH3COO-)��c(H+)��c(OH-)��c(Na+)
��5��������0.175mol/L��������Һ500mL���ɲ����������ַ�����
����һ����������ƽ��ȡ_______g��ˮ�����ƣ���������ˮ�У����500mL��Һ��
���������������Ϊ250 mL��Ũ�Ⱦ�Ϊ________�Ĵ�����������������Һ��϶��ɣ����Ϻ��������ڻ��ǰ�������֮�ͣ���
��6���������£�0.175mol/L��������Һ��PHԼΪ________(��֪�������ˮ�ⷴӦ��ƽ�ⳣ��K=Kw��Ka(CH3COOH))��
 )
)��1��д��������ˮ�ⷴӦ�Ļ�ѧ����ʽ_____________________��
��2������ͼ����˵�������Ƶ�ˮ�ⷴӦ�ﵽƽ�����_____________________��
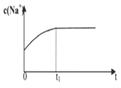 |  |  |  |
| A����Һ��c (Na��)�뷴Ӧʱ��t�Ĺ�ϵ | B.CH3COO����ˮ�������뷴Ӧʱ��t�Ĺ�ϵ | C.��Һ��PH�뷴Ӧʱ��t�Ĺ�ϵ | D.KW�뷴Ӧʱ��t�Ĺ�ϵ |
��3���ڴ�������Һ�м��������������ʣ�ˮ��ƽ��������Ӧ�����ƶ�����
A�������� B��������� C������ƹ��� D���Ȼ�粒���
��4���ڴ�������Һ�м����������������Һ����Ũ�ȵĹ�ϵʽ�ܳ�������
A��c(CH3COO-)+c(CH3COOH)��c(Na+)
B��c(Na+)+c(CH3COO-)��c(H+)��c(OH-)
C��c(CH3COO-)��c(Na+)��c(H+)��c(OH-)
D��c(CH3COO-)��c(H+)��c(OH-)��c(Na+)
��5��������0.175mol/L��������Һ500mL���ɲ����������ַ�����
����һ����������ƽ��ȡ_______g��ˮ�����ƣ���������ˮ�У����500mL��Һ��
���������������Ϊ250 mL��Ũ�Ⱦ�Ϊ________�Ĵ�����������������Һ��϶��ɣ����Ϻ��������ڻ��ǰ�������֮�ͣ���
��6���������£�0.175mol/L��������Һ��PHԼΪ________(��֪�������ˮ�ⷴӦ��ƽ�ⳣ��K=Kw��Ka(CH3COOH))��
��1��CH3COONa +H2O CH3COOH+NaOH
CH3COOH+NaOH
��2��BC(ѡ1����1�֣�����������)
��3��CD
��4��AC
��5��7.2(��7.175�ĸ�1��)��0.35mol/L����λ��1�֣�
��6��9
 CH3COOH+NaOH
CH3COOH+NaOH��2��BC(ѡ1����1�֣�����������)
��3��CD
��4��AC
��5��7.2(��7.175�ĸ�1��)��0.35mol/L����λ��1�֣�
��6��9
�����������1��������ˮ�����ɴ�����������ƣ���ѧ����ʽΪCH3COONa +H2O
 CH3COOH+NaOH
CH3COOH+NaOH��2��A�������Ӳ�ˮ�⣬����Ũ��ʼ�ղ��䣬����B����������ӿ�ʼʱˮ�����������С��ƽ��ʱ���ڱ仯����ȷ��C������ˮ������У�pH������ƽ��ʱ���ڱ仯����ȷ��D��KW��һ�¶ȳ������¶Ȳ��䣬KW���䣬����ѡBC��
��3��A�������������Һ�д���Ũ������ƽ�����ƣ�����B�����봿����壬��ƽ����ϵ������Ũ����Ӱ�죬ƽ�ⲻ�ƶ�������C���������ƹ��壬��Һ�ڴ��������Ũ������ƽ�����ƣ���ȷ��D�������Ȼ�粒��壬笠�������ˮ�����ɵ����������ӽ�ϳ�һˮ�ϰ���ʹ��Һ������������Ũ�ȼ�С��ƽ�����ƣ���ȷ����ѡCD��
��4��A����������ᣬʹ���������Ũ������������Ũ�Ȳ��䣬����A��ȷ��B���������������ᣬƽ�����ƣ����������Ũ��������������Ũ�ȣ�����C����������ᣬ����Һ�д���Ũ�Ƚϴ�ʱ������ĵ�����ڴ�������ӵ�ˮ��̶ȣ����������Ũ��������Һ�����ԣ���ȷ��D�������Ƿ����̶ȴ���ˮ��̶ȣ����������c(OH-)��c(Na+),����ѡAC��
��5����m=nM�ô����Ƶ�����Ϊ7.175g������������ƽ����������Ϊ7.2g���������������Ƶ�Ũ�ȵ������ϣ���Ϻ����ҺŨ�ȼ���Ϊ0.175mol/L������ԭ����Ũ��Ϊ0.35mol/L
��6�� �������ˮ�ⷴӦ��ƽ�ⳣ��
K=Kw��Ka(CH3COOH)=c��CH3COOH��c(OH-)/c(CH3COO-)= c(OH-)2/c(CH3COO-),����c(OH-)=10-5��Ph=9

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
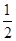 O2(g)����H����242 kJ��mol��1
O2(g)����H����242 kJ��mol��1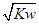 mol��L��1
mol��L��1