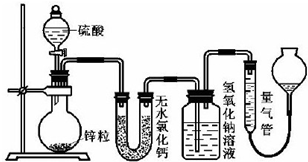
��Ŀ����
1����֪����������Ӧ����Fe+H2SO4��ϡ���TFeSO4+H2��
��Cu+2H2SO4��Ũ��$\frac{\underline{\;��\;}}{\;}$CuSO4+SO2��+2H20
�Իش��������⣺
��1����Ӧ����Fe����Ԫ�ط��ţ���Ԫ�ر���������ԭ����ΪH2����Ӧ����Cu����ԭ����SԪ�ر���ԭ��
��2������Ӧ������32g H2ʱ������H2SO4��������1568g��
��3����Ӧ��������32g S02����ʱ������H2SO4��������98g��������49g H2SO4����ԭ��ת�Ƶ���1mol��
���� ��1����Ԫ�ػ��ϼ۵ĽǶ��жϣ�������ԭ��Ӧ�У��������ڷ�Ӧ�еõ��ӱ���ԭ������Ԫ�ػ��ϼ۽��ͣ���ԭ��ʧ���ӣ�����Ԫ�ػ��ϼ����ߣ�
��2�����ݷ���ʽ�����㼴�ɣ�
��3�����ϼ۽��͵���Ԫ�����ڵ����������������ڷ�Ӧ�б���ԭ�����ݷ���ʽ�����㼴�ɣ�
��� �⣺��1����Ӧ����FeԪ�صĻ��ϼ����߱�������HԪ�ػ��ϼ۽��ͣ�����ԭ�õ���ԭ����ΪH2����Ӧ����CuԪ�صĻ��ϼ����߱�������CuΪ��ԭ����SԪ�ػ��ϼ۽��ͣ�����ԭ��
�ʴ�Ϊ��Fe��H2��Cu��S��
��2���赱��Ӧ������32g H2ʱ������H2SO4��������xg��
Fe+H2SO4��ϡ���TFeSO4+H2��
98 2
xg 32g
��x=$\frac{98��32g}{2}$=1568g��
�ʴ�Ϊ��1568��
��3��n��SO2��=$\frac{32g}{64g/mol}$=0.5mol���ɷ���ʽ��֪������2molH2SO4�μӷ�Ӧʱ����1mol����ԭ��1mol�������ԣ�����1molSO2��������0.5molSO2������1molH2SO4������Ϊ1mol��98g/mol=98g������0.5mol������������������Ϊ49g������0.5molSO2ʱ��S��+6�۽���Ϊ+4�ۣ�ת��1mol���ӣ�
�ʴ�Ϊ��98��49��1��
���� ���⿼��������ԭ��Ӧ�ļ��㣬������ѧ���ķ��������ͼ��������Ŀ��飬ע�����������ԭ��Ӧ����������Ԫ�ػ��ϼ۵ĽǶ���ʶ��ظ�������ʵ����ʣ���Ϸ�Ӧ�ķ���ʽ���㣬�ѶȲ���

| A�� | ��ɫ | B�� | ��ɫ | C�� | ��ɫ | D�� | ���ж� |
| pH��Χ | ��7 | ��7 |
| ���� | NO3- | NO��N2O��N2�е�һ�� |
| A�� | ���������£�NaNO2��NaClO��Ӧ�����ӷ���ʽΪNO2-+ClO-�TNO3-+Cl- | |
| B�� | �����NaNO2��Һ��ͨ��CO2�ɵõ�HNO2 | |
| C�� | �����NaNO2��Һ�м���ϡ����ɵõ�HNO2 | |
| D�� | �����NaNO2��Һ�м�����е��۵�����ᣬ��Һ����ɫ |
| A�� | ��ѹ�³�N2 | B�� | ��B | C�� | �����³�N2 | D�� | ��A |
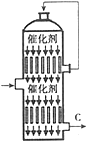 �����������ߵͳ����ں���һ�����һ�������ˮƽ�ĸߵͣ�
�����������ߵͳ����ں���һ�����һ�������ˮƽ�ĸߵͣ���1���Ի�����Ϊԭ������SO2ʱ��Ӧ�Ļ�ѧ����ʽΪ��4FeS2 +11 O2$\frac{\underline{\;����\;}}{\;}$2Fe2O3+8SO2���÷�Ӧ�ڷ���¯�н��У����豸���ƣ���
��2��ij����С����ij���Ṥ������������SO2��ƽ��ת�������ⲿ�����Ĺ�ϵ���±���ʾ�������������У����ʺϵ�����������1������ѹ��400�棻
| ѹǿ ��Pa�� �¶� ���棩 | 1.013��105 | 5.065��105 | 10.13��105 | 15.195��105 |
| 400 | 99.61% | 99.72% | 99.84% | 99.88��105 |
| 500 | 96.75% | 97.67% | 98.52% | 98.94% |
| 600 | 85.20% | 88.97% | 92.76% | 94.68% |
��4�����������У���������Ṥҵ�ľ���Ч���ص���d��
a���ʵ��ضԻ�������з���
b���ڽӴ�����ʹ���ʵ�������O2
c�����������е����ȷ����ů
d���Է�ˮ�����������������ŷ�
��5�����Ṥҵ���������Ի����к������ʣ���������ǹ�����ַ��ѡ���������������к��ɷֵĴ�����Ҫ��ֿ��ǻ�����Ҫ��
��ij�мƻ���һ�����Ṥ������ַ��A��B��C�����ط��ɹ�ѡ�������ոó�����B�أ�����Ϊѡ��B�ؽ����������ǣ����ٻش����㣩B��Զ��������������ġ�B��Զ����Ʒ���ܵĵط���
�ڽ�β����SO2��һ��������ת��ΪCaSO4��Ȼ�����ý�̿����ת��ΪCaS������һ�ֿ�ȼ���������ɣ�������Ҫ�����壬д��CaSO4ת��ΪCaS�Ļ�ѧ����ʽCaSO4+4C$\frac{\underline{\;����\;}}{\;}$CaS+4CO��
��6�����õ���������ǣ�FeS2�������ᣬ����ԭ�ϵ��������ʾ�Ϊ90%������ߵõ�������������Ϊ15��8��
| A�� | ���Ԫ����ͬ�Ҹ�Ԫ����������Ҳ��ͬ��һ����Ϊͬ���칹�� | |
| B�� | ��Է���������ͬ���л���һ��Ϊͬ���칹�� | |
| C�� | ����ͬ�ķ���ͨʽ�ҷ�����������һ�������ɸ�CH2ԭ���ŵ�����һ����Ϊͬϵ�� | |
| D�� | �ṹ��ͬ���������ƣ���ѧʽ��ͬ�����ʻ���ͬ���칹�� |
| A�� | pH=4�������У�c��H+��=4.0mol•L-1 | |
| B�� | NH4Cl��Һ�У�c��Cl-��=c��NH4+�� | |
| C�� | NaCl��Һ�У�c��Na+��+c��H+��=c��Cl-��+c��OH-�� | |
| D�� | Na2CO3��Һ�У�c��HCO3-��+c��H2CO3��+c��CO32-��=2c��Na+�� |