��Ŀ����
̫��������ϵ�м�Ϊ��ͨ��һ�ź��ǣ����ҵ��Ⱥ˷�Ӧʹ֮���ϵ��������������̫��������������ּ��������Ⲩ�Σ��ɼ��Ⲩ�κͺ���Ⲩ�Σ����ǵ������ֱ�ռ�ܷ�������9%��44%��47%������Ĵ������У���������ijɷ�Ϊ��������벵ȣ�ռ����������99.96%���ɱ�����ɷ���Ҫ��CO2��ˮ���ͳ����ȣ���Щ����ĺ�����С�����Դ�������״����Ӱ�켫����������һ����ָ�߶��������10~15Km�Ĵ����㣬���г�����Ũ�Ⱥܵͣ������ۺϳɱ�״���������ۻ����Ҳ����0.3cm���京����С�����Ե�������������Ӱ��ܴ�
��1������ѹǿP������߶�Z��m�����������С�����鹫ʽΪPZ=![]() ������P0Ϊ�ر�����ѹֵ��e=2.718���������㼯����24km�ĸ߿մ������ڸø߶ȴ����¶�Ϊ��50�档�Թ��������ĺ�ȡ�
������P0Ϊ�ر�����ѹֵ��e=2.718���������㼯����24km�ĸ߿մ������ڸø߶ȴ����¶�Ϊ��50�档�Թ��������ĺ�ȡ�
��2����Ҫ˵��������Ե�������Ȧ�����塣
![]() ��3����������������Լ����µķ�Ӧ���̣��߲�����е�һ����Ҫ�Ĺ⻯ѧ��Ӧ������������̫�������в�����<0.24��m�Ĺ��ӣ����Ϊ��ԭ�ӣ���O2+h�ͣ���<0.24��m�� O+O���ɴ˿��γ�һϵ�еķ�Ӧ����������Ҫ������ԭ�Ӻ��������ڵ����壨M���IJ������γɳ������䷴Ӧ����ʽΪ �������M��Ҫ�������ӻ��ӣ��������ڷ�Ӧ������ͬʱ���������غ�Ͷ����غ�������ġ������ڦ�<0.18��m�ķ��������£������������Ӻ���ԭ�ӣ��䷴Ӧ����ʽΪ ��
��3����������������Լ����µķ�Ӧ���̣��߲�����е�һ����Ҫ�Ĺ⻯ѧ��Ӧ������������̫�������в�����<0.24��m�Ĺ��ӣ����Ϊ��ԭ�ӣ���O2+h�ͣ���<0.24��m�� O+O���ɴ˿��γ�һϵ�еķ�Ӧ����������Ҫ������ԭ�Ӻ��������ڵ����壨M���IJ������γɳ������䷴Ӧ����ʽΪ �������M��Ҫ�������ӻ��ӣ��������ڷ�Ӧ������ͬʱ���������غ�Ͷ����غ�������ġ������ڦ�<0.18��m�ķ��������£������������Ӻ���ԭ�ӣ��䷴Ӧ����ʽΪ ��
��4������Ļ��������һЩ���壬�ܶԴ����еij������ƻ����á�ʹ�京�����٣���������غ����ֱ����в���������������档��Щ�������������ϼ��������������س��ֳ����ն��ı������������Ǽ���Ĺ�ע����Ҫ˵��������������Щ����Դ��������������ƻ����á�
��1��5cm
��2�������������߶��������棬�ɴٽ������ĸƻ���Ԥ�����Ͳ����������������Ŀ��������������������������ƻ����ﵰ�ͻ������ʣ����ϸ����������ʹƤ��������ߡ�ɫ�س�����Ƥ�������������ӣ����˺��۾��������ۼ��Խ�Ĥ�ס������ϡ���ʧ��������������������������ã����赲�������������߷��䵽�ر����Ե���������������ã���ˣ�������Ĵ��ڶԵ��������������Ҫ��
![]()
![]() ��3��O2+O+M O3+M O3+ h�ͣ���<0.18��m�� O2+O
��3��O2+O+M O3+M O3+ h�ͣ���<0.18��m�� O2+O
��4�������в��������壺�������ɻ��ŷŴ�����NO��һЩũҩ��ɱ������ܲ���N2O����Ϊ�����������ϵͳ�еĹ㷺ʹ�õķ��ﰺ����Щ��������ͷ��ﰺ�ȶԳ����㶼�����ƻ����á�
����:
��������˳�������й�֪ʶ�������˳��������ɺ����ġ�����������ȵ���Ϣ��Ҫ��ѧ���ܹ�����ʾ��Ϣ����ȡ���������ص���Ϣ���ų���һЩ�����������⣬��Ҫ��ѧ���ܹ��Ը���������м��Ӷ����п�ѧ�Ĺ��㡣����Ŀ�ṩ�Ĺ�ʽ��PZ=![]() �������Z=24km�߿մ�����ѹPZ=
�������Z=24km�߿մ�����ѹPZ=![]() =
= ![]() = P0�� e-3=0.05P0������̬����
= P0�� e-3=0.05P0������̬����![]() ������
������![]() ����ΪD0��D<<Z�����У�
����ΪD0��D<<Z�����У�![]() ���ɴ˿ɵã�D=
���ɴ˿ɵã�D=![]() ��
��

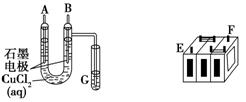
 ���ſ����ϵ�д�
���ſ����ϵ�д� ���Ŀ����ϵ�д�
���Ŀ����ϵ�д� ������ӱ������ͯ������ϵ�д�
������ӱ������ͯ������ϵ�д� 2NH3(g)����H����92 kJ��mol��1����ش��������⣺
2NH3(g)����H����92 kJ��mol��1����ش��������⣺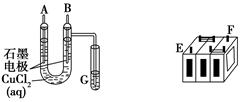
 2NH3(g)����H����92 kJ��mol��1����ش��������⣺
2NH3(g)����H����92 kJ��mol��1����ش��������⣺