��Ŀ����
����Ŀ����12���� ������������־����־�����������й��ڰ�ͭ�ļ��أ���������ͭ��ͭ���Ͻ����������⣬����Ҫ������ң������������������Ʒ���ش��������⣺
��1����Ԫ�ػ�̬ԭ�ӵĵ����Ų�ʽΪ_________��3d�ܼ��ϵ�δ�ɶԵĵ�����Ϊ______��
��2�����������ڰ�ˮ�γ�[Ni��NH3��6]SO4��ɫ��Һ��
��[Ni��NH3��6]SO4�������ӵ����幹����_____��
����[Ni��NH3��6]2+��Ni2+��NH3֮���γɵĻ�ѧ����Ϊ_ _���ṩ�µ��ӶԵijɼ�ԭ����__ ___��
�����ķе�_____�������ڡ����ڡ������PH3����ԭ����____ ������____ _����������ԡ��Ǽ��ԡ���������ԭ�ӵĹ���ӻ�����Ϊ____��
��3������ͭ����������______���γɵľ��壺Ԫ��ͬ�����ĵڶ������ֱܷ�Ϊ��I��Cu��=1959kJ/mol��I��Ni��=1753kJ/mol��I��Cu����I��Ni����ԭ����______��
��4��ij����ͭ�Ͻ�����������ṹ��ͼ��ʾ��
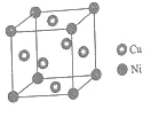
������ͭԭ������ԭ�ӵ�������Ϊ_____��
���𰸡���1�� 1S22S22P63S23P63d84S2����[Ar]3d84S2 2 ��2�� ��������
����λ�� N ������ NH3���Ӽ���γ���� ���Է��� SP3
��3�������� Cu+�����Ų��ʰ����״̬���Ƚ��ȶ���ʧ������Ҫ�����ߣ��ڶ���������ֵ��
��4��3:1
��������
�����������1��NiԪ��ԭ�Ӻ��������Ϊ28����������Ų�ʽΪ1s22s22p63s23p63d84s2��3d�ܼ��ϵ�δ�ɶԵ�����Ϊ2��
��2����SO42-��Sԭ�ӵŵ��Ӷ���=![]() =0���۲���Ӷ���=4+0=4�����ӿռ乹��Ϊ�������壻
=0���۲���Ӷ���=4+0=4�����ӿռ乹��Ϊ�������壻
��Ni2+�ṩ�չ����NH3��Nԭ�Ӻ��йµ��Ӷԣ�����֮���γ���λ����
��PH3����֮��Ϊ���»�������������֮���γ���������Ӽ���������ǿ�����������ʵķе㣬�ʰ����ķе����PH3���ӵģ�NH3����Ϊ�����νṹ������������������IJ��غϣ����ڼ��Է��ӣ�Nԭ����1�Թ¶Ե��ӣ��γ�3��N-H�����ӻ������ĿΪ4����ԭ�Ӳ�ȡsp3�ӻ���
��3������ͭ���������ڽ������壬�����ɽ������γɵľ��壻Cu+����Χ�����Ų�Ϊ3d10��Ni+����Χ�����Ų�Ϊ3d84s1��Cu+�ĺ�������Ų����ȶ���ʧȥ�ڶ������Ӹ��ѣ�Ԫ��ͭ�ĵڶ������ܸ������ģ�
��4����������Ni���ڶ��㣬Cu�������ģ�����Niԭ����ĿΪ8��![]() =1��Cuԭ����Ŀ=6��
=1��Cuԭ����Ŀ=6��![]() =3����Cu��Niԭ����Ŀ֮��Ϊ3��1��
=3����Cu��Niԭ����Ŀ֮��Ϊ3��1��

 ������ÿ�ʱ�Ż���ҵϵ�д�
������ÿ�ʱ�Ż���ҵϵ�д�

