��Ŀ����
ij��ѧ��һ������ȤС��Ϊ̽��ͭ������ķ�Ӧ������������ͼ��ʾװ�ý����й�ʵ�飮
��1���ȹرջ���a����6.4gͭƬ��12mLijŨ�ȵ�Ũ�������Բ����ƿ�й�������Ӧ��ϣ�������ƿ�л���ͭƬʣ�࣮�ٴ���a���������е�������������Բ����ƿ�����ͭƬ��ȫ��ʧ��
д��������������ƿ�ڷ����Ļ�ѧ����ʽ��
�رջ���a______��
����a______��
��2����С���ͬѧ�ԡ���μ���SO2�л�������CO2���������ܸ���Ȥ������A��ͭƬ����ľ̿�ۣ�����A��B֮������������װ�ã�
�Լ���a��NaOH��Һ b��Ʒ����Һ c������KMnO4��Һ d��Ca��OH��2��Һ
��ش�
�ٸ�ͬѧ��ʵ��װ��A�з����Ļ�ѧ����ʽ��______��
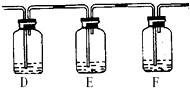
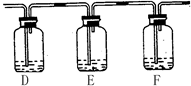
����Ҫ�ﵽ��Ŀ�ģ������ڣ��������ṩ�Լ���ţ�
D���______��E���______��F���______��
��3����ʵ֤ʵ���ڣ�1����ͭƬ��ȫ��ʧ����������ʣ�࣬��ͬѧ���ⶨ��������ʵ���Ũ�ȣ����跴Ӧǰ����Һ����仯���Բ��ƣ�����Ӧ����Һ�м��뺬����a mol��NaOH��Һ�պ�ʹ��Һ��Cu2+ȫ���������ݴˣ����������������ʵ���Ũ�������ܣ���д���������ʵ���Ũ�ȵı���ʽ��______mol/L���ú�a�Ĵ���ʽ��������ܣ��ÿղ����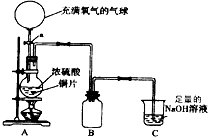
��1���ȹرջ���a����6.4gͭƬ��12mLijŨ�ȵ�Ũ�������Բ����ƿ�й�������Ӧ��ϣ�������ƿ�л���ͭƬʣ�࣮�ٴ���a���������е�������������Բ����ƿ�����ͭƬ��ȫ��ʧ��
д��������������ƿ�ڷ����Ļ�ѧ����ʽ��
�رջ���a______��
����a______��
��2����С���ͬѧ�ԡ���μ���SO2�л�������CO2���������ܸ���Ȥ������A��ͭƬ����ľ̿�ۣ�����A��B֮������������װ�ã�

�Լ���a��NaOH��Һ b��Ʒ����Һ c������KMnO4��Һ d��Ca��OH��2��Һ
��ش�
�ٸ�ͬѧ��ʵ��װ��A�з����Ļ�ѧ����ʽ��______��
����Ҫ�ﵽ��Ŀ�ģ������ڣ��������ṩ�Լ���ţ�
D���______��E���______��F���______��
��3����ʵ֤ʵ���ڣ�1����ͭƬ��ȫ��ʧ����������ʣ�࣬��ͬѧ���ⶨ��������ʵ���Ũ�ȣ����跴Ӧǰ����Һ����仯���Բ��ƣ�����Ӧ����Һ�м��뺬����a mol��NaOH��Һ�պ�ʹ��Һ��Cu2+ȫ���������ݴˣ����������������ʵ���Ũ�������ܣ���д���������ʵ���Ũ�ȵı���ʽ��______mol/L���ú�a�Ĵ���ʽ��������ܣ��ÿղ����

��1��Ũ�������ǿ�����ԣ��ڼ���ʱ������������ͭ����������ͭ�����������ˮ������ʽΪ2H2SO4��Ũ��+Cu
CuSO4+2H2O+2SO2����ͭ��Ũ���ᷴӦ��һ�����̷�Ӧ�����ŷ�Ӧ���У�����Ũ�Ƚ��ͣ�ϡ������ͭ����Ӧ����ͨ���������������ܼ�����ͭ��Ӧ����Ӧ����ʽΪ��2Cu+O2+2H2SO4
2CuSO4+2H2O
����ֲ�д�ɣ�2Cu+O2=2CuO��CuO+H2SO4=CuSO4+H2OҲ�ɣ���
�ʴ�Ϊ��Cu+2H2SO4��Ũ��
CuSO4+SO2��+2H2O��2Cu+O2+2H2SO4
2CuSO4+2H2O����ֲ�д�ɣ�2Cu+O2=2CuO��CuO+H2SO4=CuSO4+H2OҲ�ɣ���
��2����Ũ�����ڼ��ȵ�������C��Ӧ����SO2��CO2����Ӧ�ķ���ʽΪC+2H2SO4��Ũ��
CO2��+2SO2��+2H2O��
�ʴ�Ϊ��C+2H2SO4��Ũ��
CO2��+2SO2��+2H2O��
�ڼ���SO2�л�������CO2������SO2�ľ��л�ԭ�ԣ��Ƚ��������ͨ�����Ը��������Һ������Ʒ�����SO2�Ƿ���������ͨ�����ʯ��ˮ�۲���Һ�Ƿ����ǣ�
�ʴ�Ϊ��c��b��d��
��3��n��Cu��=
=0.1mol��
��n��CuSO4��=0.1mol��
�跴Ӧ�����Һ�к���xmolH2SO4��
��H2SO4+2NaOH=Na2SO4+2H2O
1 2
x 2x
CuSO4+2NaOH=Na2SO4+Cu��OH��2��
1 2
0.1mol 0.2mol
����2x+0.2=a
x=
���ԣ���Ӧ����������ʵ���Ũ��Ϊ��
=
mol/L��
�ʴ�Ϊ���ܣ�
��
| ||
| ||
����ֲ�д�ɣ�2Cu+O2=2CuO��CuO+H2SO4=CuSO4+H2OҲ�ɣ���
�ʴ�Ϊ��Cu+2H2SO4��Ũ��
| ||
| ||
��2����Ũ�����ڼ��ȵ�������C��Ӧ����SO2��CO2����Ӧ�ķ���ʽΪC+2H2SO4��Ũ��
| ||
�ʴ�Ϊ��C+2H2SO4��Ũ��
| ||
�ڼ���SO2�л�������CO2������SO2�ľ��л�ԭ�ԣ��Ƚ��������ͨ�����Ը��������Һ������Ʒ�����SO2�Ƿ���������ͨ�����ʯ��ˮ�۲���Һ�Ƿ����ǣ�
�ʴ�Ϊ��c��b��d��
��3��n��Cu��=
| 6.4g |
| 64g/mol |
��n��CuSO4��=0.1mol��
�跴Ӧ�����Һ�к���xmolH2SO4��
��H2SO4+2NaOH=Na2SO4+2H2O
1 2
x 2x
CuSO4+2NaOH=Na2SO4+Cu��OH��2��
1 2
0.1mol 0.2mol
����2x+0.2=a
x=
| a-0.2 |
| 2 |
���ԣ���Ӧ����������ʵ���Ũ��Ϊ��
| ||
| 0.012L |
| 125(a-0.2) |
| 3 |
�ʴ�Ϊ���ܣ�
| 125(a-0.2) |
| 3 |

��ϰ��ϵ�д�
 ��˼ά������ҵ���ټ��ִ�ѧ������ϵ�д�
��˼ά������ҵ���ټ��ִ�ѧ������ϵ�д�
�����Ŀ
 ij��ѧ��һ������ȤС��Ϊ̽��ͭ������ķ�Ӧ������������ͼ��ʾװ�ý����й�ʵ�飮
ij��ѧ��һ������ȤС��Ϊ̽��ͭ������ķ�Ӧ������������ͼ��ʾװ�ý����й�ʵ�飮 �Լ���a��NaOH��Һ b��Ʒ����Һ c������KMnO4��Һ d��Ca��OH��2��Һ
�Լ���a��NaOH��Һ b��Ʒ����Һ c������KMnO4��Һ d��Ca��OH��2��Һ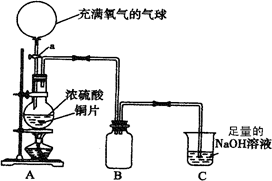
 ��
�� ��NaOH��Һ�պ�ʹ��Һ��Cu2+ȫ���������ݴˣ����������������ʵ���Ũ�������ܣ���д���������ʵ���Ũ�ȵı���ʽ�� mol/L(
��NaOH��Һ�պ�ʹ��Һ��Cu2+ȫ���������ݴˣ����������������ʵ���Ũ�������ܣ���д���������ʵ���Ũ�ȵı���ʽ�� mol/L( �ú�a�Ĵ���ʽ��������ܣ��ÿղ���)��
�ú�a�Ĵ���ʽ��������ܣ��ÿղ���)��


