��Ŀ����
ͼ��X��Y��ZΪ���ʣ�����Ϊ���������֮�������ͼת����ϵ�����ֲ�������ȥ�������У�A�׳ƴ�����������E�Dz�����ˮ�������������������ᷴӦ��
�ش��������⣺
��1����ɵ���Y��Ԫ�������ڱ��е�λ����______��M�д��ڵĻ�ѧ������Ϊ______��R�Ļ�ѧʽ��______��
��2��һ�������£�Z��H2��Ӧ����ZH4��ZH4�ĵ���ʽΪ______��
��3����֪A��1molAl��Ӧת��ΪXʱ���������ʾ�Ϊ���壩���ų�aKJ������д���÷�Ӧ���Ȼ�ѧ����ʽ��______��
��4��д��A��D��ϡ��Һ��Ӧ����G�����ӷ���ʽ��______��
���𰸡����������������Ϣ�����ת����ϵ�ͷ�Ӧ�����ķ����ƶϵõ���A�׳ƴ�������������Ϊ��������������������ᷴӦ�Ҳ�����ˮ��������������SiO2����EΪSiO2�����ݿ�ͼ�е�ת����ϵ����֪XΪ����YΪO2��ZΪSi��DΪ���ᡢMΪ�����ơ�GΪ��������RΪH2SiO3��
��1������ԭ�Ӻ�������Ų���ȷ��Ԫ�������ڱ��е�λ�ã����������еijɼ�Ԫ��ȷ�������еĻ�ѧ�����ͣ�
��2�����ݵ���ʽ����д�����������л�ѧ�����������
��3�������Ȼ�ѧ����ʽ�ĺ������д������������
��4�����������ǿ�����Ժ������������еĶ�����Ԫ�ؾ��л�ԭ�Ե���������д��
����⣺A�׳ƴ�������������Ϊ��������������������ᷴӦ�Ҳ�����ˮ��������������SiO2����EΪSiO2�����ݿ�ͼ�е�ת����ϵ����֪XΪ����YΪO2��ZΪSi��DΪ���ᡢMΪ�����ơ�GΪ��������RΪH2SiO3��
��1��������ԭ�ӵĺ�������Ų���֪��Ԫ�������ڱ��ĵڶ����ڵ�VIA�壻�������������Ӽ������ۼ���R�Ļ�ѧʽ��
H2SiO3����H4SiO4�����ʴ�Ϊ���ڶ����ڵ�VIA�壻���Ӽ������ۼ��� H2SiO3����H4SiO4����
��2��ZH4�ķ���ʽΪSiH4�������ʽΪ��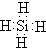 ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
��3�������Ȼ�ѧ����ʽ����д��������дԭ��Ӧ���Ȼ�ѧ����ʽΪ8Al��s��+3Fe3O4��s��=9Fe��s��+4Al2O3��s����H=-8a kJ/mol��
�ʴ�Ϊ��8Al��s��+3Fe3O4��s��=9Fe��s��+4Al2O3��s����H=-8a kJ/mol��
��4�����������������ᷴӦ���������еĶ�����Ԫ�ر���������ۣ������е�Ԫ�ر���ԭ��+2�ۣ���Ӧ�����ӷ���ʽΪ��3Fe3O4+28H++NO3-=9Fe3++NO��+14H2O��
�ʴ�Ϊ��3Fe3O4+28H++NO3-=9Fe3++NO��+14H2O
����������Ŀ��һ����ͼ�ƶ��⣬���������Ϣ�Ƴ����������Ǵ����ǰ�ᣬȻ������ѧ֪ʶ���ش�
��1������ԭ�Ӻ�������Ų���ȷ��Ԫ�������ڱ��е�λ�ã����������еijɼ�Ԫ��ȷ�������еĻ�ѧ�����ͣ�
��2�����ݵ���ʽ����д�����������л�ѧ�����������
��3�������Ȼ�ѧ����ʽ�ĺ������д������������
��4�����������ǿ�����Ժ������������еĶ�����Ԫ�ؾ��л�ԭ�Ե���������д��
����⣺A�׳ƴ�������������Ϊ��������������������ᷴӦ�Ҳ�����ˮ��������������SiO2����EΪSiO2�����ݿ�ͼ�е�ת����ϵ����֪XΪ����YΪO2��ZΪSi��DΪ���ᡢMΪ�����ơ�GΪ��������RΪH2SiO3��
��1��������ԭ�ӵĺ�������Ų���֪��Ԫ�������ڱ��ĵڶ����ڵ�VIA�壻�������������Ӽ������ۼ���R�Ļ�ѧʽ��
H2SiO3����H4SiO4�����ʴ�Ϊ���ڶ����ڵ�VIA�壻���Ӽ������ۼ��� H2SiO3����H4SiO4����
��2��ZH4�ķ���ʽΪSiH4�������ʽΪ��
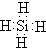 ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
����3�������Ȼ�ѧ����ʽ����д��������дԭ��Ӧ���Ȼ�ѧ����ʽΪ8Al��s��+3Fe3O4��s��=9Fe��s��+4Al2O3��s����H=-8a kJ/mol��
�ʴ�Ϊ��8Al��s��+3Fe3O4��s��=9Fe��s��+4Al2O3��s����H=-8a kJ/mol��
��4�����������������ᷴӦ���������еĶ�����Ԫ�ر���������ۣ������е�Ԫ�ر���ԭ��+2�ۣ���Ӧ�����ӷ���ʽΪ��3Fe3O4+28H++NO3-=9Fe3++NO��+14H2O��
�ʴ�Ϊ��3Fe3O4+28H++NO3-=9Fe3++NO��+14H2O
����������Ŀ��һ����ͼ�ƶ��⣬���������Ϣ�Ƴ����������Ǵ����ǰ�ᣬȻ������ѧ֪ʶ���ش�

��ϰ��ϵ�д�
 ��Ȥ������ҵ���ϿƼ�������ϵ�д�
��Ȥ������ҵ���ϿƼ�������ϵ�д�
�����Ŀ

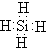

 9Fe3++NO��+14H2O
9Fe3++NO��+14H2O