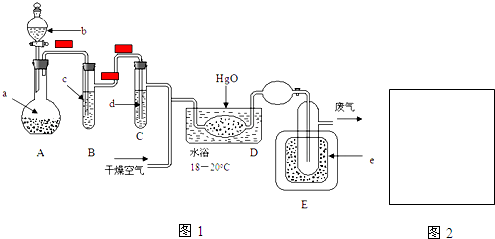
��Ŀ����
9���������ȣ�ClO2����һ����ˮ�����ȷ����й㷺Ӧ�õĸ�Ч��ȫ��������ClO2��һ�ֻ���ɫ�����壬������ˮ��ʵ������NH4Cl�����ᡢNaClO2���������ƣ�Ϊԭ���Ʊ�ClO2��������ͼ1��
��1��д�����ʱ������Ӧ�Ļ�ѧ����ʽ��NH4Cl+2HCl$\frac{\underline{\;���\;}}{\;}$3H2��+NCl3��
��2����ȥClO2�е�NH3��ѡ�õ��Լ���C��������ţ�
A��̼������Һ B����ʯ�� C��Ũ���� D��ˮ
��3���ⶨClO2����ͼ2���Ĺ������£�����ƿ�м��������ĵ⻯�أ���100mLˮ�ܽ���ټ�3mL������Һ���ڲ���Һ����м���ˮ�������ɵ�ClO2����ͨ����������ƿ�б����գ�����������е�ˮ��Һ������ƿ�У����뼸�ε�����Һ����cmol/L��������Ʊ���Һ�ζ� ��I2+2S2O32-�T2I-+S4O62-��������ȥVmL�����������Һ��
��ClO2ͨ����ƿ�����Ե⻯����Һ��Ӧ����ԭ����ΪCl-������Ӧ�����ӷ���ʽΪ��2ClO2+10I-+8H+=2Cl-+5I2+4H2O��
��װ���в���Һ��ܵ���������ˮ�ٴ����ղ���Ķ����������壬��ʹ��ƿ����ѹǿ��ȣ�
�۵ζ����յ����������Һ����ɫǡ�ñ�Ϊ��ɫ���Ұ�����ڲ���ɫ��
�ܲ��ClO2������m��ClO2��1.35cv��10-2g�����ú�c��V�Ĵ���ʽ��ʾ��
���� ��1���ɹ�������ת����ϵ��֪������Ȼ������������Һ������NCl3��H2��
��2������Ϣ��֪��ClO2������ˮ�����Բ�������ˮ��Һ���գ�����Ϊ�������壮�ݴ��жϣ�
��3��������Ŀ��Ϣ��֪��ClO2ͨ����ƿ�����Ե⻯����Һ��Ӧ������I-ΪI2����������ԭΪCl-��ͬʱ����ˮ��
����ˮ�ٴ����ղ���Ķ����������壬��ʹ��ƿ����ѹǿ��ȣ�
����Һ����ɫǡ�ñ�Ϊ��ɫ���Ұ�����ڲ���ɫ��˵���ζ����յ㣻
�ܸ��ݹ�ϵʽ2ClO2��5I2��10Na2S2O3����n��ClO2�����ٸ���m=nM����m��ClO2����
��� �⣺��1���ɹ�������ת����ϵ��֪������Ȼ������������Һ������NCl3��H2����Ӧ����ʽΪNH4Cl+2HCl$\frac{\underline{\;���\;}}{\;}$3H2��+NCl3��
�ʴ�Ϊ��NH4Cl+2HCl$\frac{\underline{\;���\;}}{\;}$3H2��+NCl3��
��2��A��ClO2������ˮ����������̼������Һ���հ�������A����
B����ʯ�Ҳ������հ�������B����
C��Ũ����������հ������Ҳ�Ӱ��ClO2����C��ȷ��
D��ClO2������ˮ����������ˮ���հ�������D����
��ѡ��C��
��3��������Ŀ��Ϣ��֪��ClO2ͨ����ƿ�����Ե⻯����Һ��Ӧ������I-ΪI2����������ԭΪCl-��ͬʱ����ˮ����Ӧ���ӷ���ʽΪ2ClO2+10I-+8H+=2Cl-+5I2+4H2O��
�ʴ�Ϊ��2ClO2+10I-+8H+=2Cl-+5I2+4H2O��
��װ���в���Һ��ܵ������ǣ���ˮ�ٴ����ղ���Ķ����������壬��ʹ��ƿ����ѹǿ��ȣ�
�ʴ�Ϊ����ˮ�ٴ����ղ���Ķ����������壬��ʹ��ƿ����ѹǿ��ȣ�
����Һ����ɫǡ�ñ�Ϊ��ɫ���Ұ�����ڲ���ɫ��˵���ζ����յ㣬
�ʴ�Ϊ����Һ����ɫǡ�ñ�Ϊ��ɫ���Ұ�����ڲ���ɫ��
��VmLNa2S2O3��Һ����Na2S2O3���ʵ���ΪV•10-3 L��cmol/L=c•V•10-3 mol����
���ݹ�ϵʽ��2ClO2��5I2��10Na2S2O3��
2 10
n��ClO2�� c•V•10-3 mol
����n��ClO2��=$\frac{1}{5}$c•V•10-3 mol��
����m��ClO2��=$\frac{1}{5}$c•V•10-3 mol��67.5g/mol=1.35cv��10-2g��
�ʴ�Ϊ��1.35cv��10-2g��
���� ���⿼���Ķ���Ŀ��ȡ��Ϣ������������ԭ��Ӧ�ζ���Ӧ�á����Ӽ��顢�Թ������̼�װ����������ȣ��Ѷ��еȣ�Ҫ��ѧ��Ҫ����ʵ��ʵ�����֪ʶ�����Ӧ����Ϣ��������������ע�����֪ʶ��ȫ�����գ�

| A�� | c��OH-�������ʵ���Ũ�� | B�� | c��CH3COO-�������ʵ���Ũ�� | ||
| C�� | c ��CH3COOH��/c��CH3COO-�� | D�� | c��H+��•c��OH-�� |
| A�� | ����ʯ�����ᷴӦ��CO32-+2H+�TH2O+CO2�� | |
| B�� | ����CO2�����ʯ��ˮ��Ӧ��Ca2++2OH-+CO2�TCaCO3��+H2O | |
| C�� | NH4HSO3������NaOH��Ӧ��NH4++HSO3-+20H-�TNH3•H2O+SO32-+H2O | |
| D�� | ��NH4��2SO4��Ba��OH��2��Ӧ��2NH4++SO42-+Ba2++2OH-�TBaSO4��+2NH3•H2O |
| A�� | NH4+��Cu2+��Cl-��NO3- | B�� | K+��Fe3+��OH-��SCN- | ||
| C�� | Ag+��NO3-��Cl-��K+ | D�� | Na+��CH3COO-��H+��OH- |
| A�� | Fe | B�� | Ag | C�� | Al | D�� | Na |