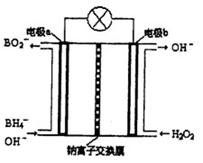
��Ŀ����
�״����ӽ���Ĥȼ�ϵ���н��״�����ת��Ϊ���������ַ�Ӧԭ����
��CH3OH(g)��H2O(g)=CO2(g)��3H2(g)����H1����49.0 kJ/mol
��CH3OH(g)��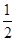 O2(g)=CO2(g)��2H2(g)����H2����192.9 kJ/mol
O2(g)=CO2(g)��2H2(g)����H2����192.9 kJ/mol
����˵����ȷ����(����)
��CH3OH(g)��H2O(g)=CO2(g)��3H2(g)����H1����49.0 kJ/mol
��CH3OH(g)��
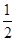 O2(g)=CO2(g)��2H2(g)����H2����192.9 kJ/mol
O2(g)=CO2(g)��2H2(g)����H2����192.9 kJ/mol����˵����ȷ����(����)
| A��1 mol CH3OH��ȫȼ�շų�����192.9 kJ |
B�����е������仯��ͼ��ʾ����Q��E3��E1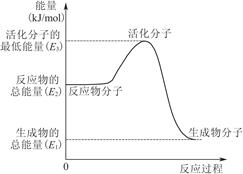 |
| C��H2ȼ���ܷų��������ȣ���CH3OHת���H2�Ĺ��̱����������� |
| D�����ݢ���֪����25 �棬101 kPaʱ��1 mol CH3OH(g)��ȫȼ������CO2��H2O�ų�������Ӧ����192.9 kJ |
D
��Ӧ�ڲ��Ǽ״�����ȫȼ�գ�A������е������仯ӦΪͼ��E2��E1��B������ɷ�Ӧ�ڣ�CH3OHת���H2Ϊ���ȹ��̣�C�����H2ȼ������H2Oʱ��Ҫ�ų���������D����ȷ

��ϰ��ϵ�д�
 ���ٴ���������ѧϰ����ѧ�ں����ν�ϵ�д�
���ٴ���������ѧϰ����ѧ�ں����ν�ϵ�д�
�����Ŀ
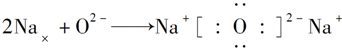
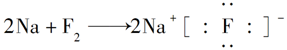
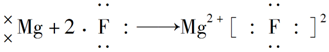
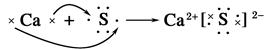
 2H2O(g)����H="-483.6" kJ/mol,��������ȼ����Ϊ241.8 kJ
2H2O(g)����H="-483.6" kJ/mol,��������ȼ����Ϊ241.8 kJ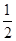 O2��g��=CO2��g����2H2��g��
O2��g��=CO2��g����2H2��g��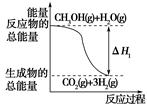
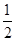 O2(g)=CO(g)��H����393.5 kJ/mol
O2(g)=CO(g)��H����393.5 kJ/mol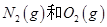 ��Ӧ����
��Ӧ����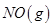 �����е������仯������˵����ȷ����
�����е������仯������˵����ȷ����
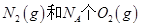 ��Ӧ�ų�������Ϊ180kJ
��Ӧ�ų�������Ϊ180kJ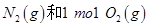 ���е�������С��2mol
���е�������С��2mol  ����ȡ�״����䷴ӦΪ��CO2+3H2
����ȡ�״����䷴ӦΪ��CO2+3H2 CH3OH+H2O ���³�ѹ����֪���з�Ӧ�������仯��ͼʾ��
CH3OH+H2O ���³�ѹ����֪���з�Ӧ�������仯��ͼʾ��