��Ŀ����
���л�ѧѧϰ�Σ����Ĵ�������ӦҲ����ij����M�����ԭ��������100������������������MԪ�ص�������һ������������NH3��±���ӵȰ�ij�̶��������ν�ϳ��ȶ�����������ӣ�������ԭ���ţ���
��1��150�桢��ѹ�£�6.08g����������0.6mol HCl�����ַ������ֽⷴӦ����������ʣ�࣬�����Ϊ0.48mol���ý���M�����ԭ������Ϊ___________��
��������Ӧ���ù�������ˮ���50.0mL��Һ������Һ�����ʵ����ʵ���Ũ��Ϊ
_______mol��L-1��
��2��ȡ��1����������Һ12.5mL��ϡ����25mL������ͨ��2688mL����������£�����һ�������·�Ӧǡ����ȫ���õ�����B��Ħ������Ϊ260.5g/mol����������1.5mol/L��AgNO3��Һ�ζ����ﵽ�յ�ʱ����ȥAgNO3��Һ40.0mL����BͶ������ռ���Һ�У�δ����NH3���ݳ�����B�Ļ�ѧʽ�ɱ�ʾΪ ��
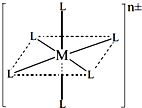
��3����֪����ͼ�У�L��λ����ȫ��ͬ��������һ��������[M(NH3)6-xClx]n����1��x��5����xΪ���������ṹ������ͼ��
����������ӹ���2�ֲ�ͬ�ṹ���������ӵ�ʽ��Ϊ ��
��4��һ�������£�3.04g�ý���������ǡ�ñ�ij��������Ч���൱��0.03mol O2���������ټ���0.05mol KCl����һЩ����մ�����K��M��Ԫ��ǡ����ȫ�γɺ������Σ�ʽ����360���������ڲ��������Ӹ�����Ϊ1:2������ε�ʽ���Լ����ʵ����ֱ�Ϊ_______________��
��1��52�� ��2�֣�
1.6 ��1�֣�
��2��[Cr(NH3)6]Cl 3 ��2�֣�
3 ��2�֣�
��3��191.0��209.5��228.0 ��3�֣�
��4��294��0.015mol�� ��1�֣�
194��0.01mol ��1�֣�
����

 ���������������Բ��������ϵ�д�
���������������Բ��������ϵ�д�