��Ŀ����
���и���������ָ����Һ�У��ܴ����������
��������Һ�У�Fe3+��Al3+��NO3����I����Cl��
��pH=11����Һ�У�CO32����Na+��AlO2����NO3����SO32��
����ˮ�����c(H+)=10��12mol��L��1����Һ�У�Cl����K+��NO3����NH4+��S2O32��
�ܼ������ָʾ�����Ժ�ɫ����Һ�У�Mg2+��NH4+��Cl����K+��SO42��
| A���٢� | B���٢� | C���ڢ� | D���ۢ� |
C
���������������Fe3+��I-�����棬���ڿ��Դ������ڣ���ȷ���۸���Һ����������Ҳ�����Ǽ��ԣ������ԣ���S2O32-���������ڣ����ܼ����Ժ�ɫ����ҺΪ���ԣ����ӿ��Դ������棬��ȷ��
���㣺���⿼�������ӹ���֪ʶ��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д����и��������ڸ���������һ���ܴ����������
| A��ˮ�������c��H+��=1��10-14 mol/L����Һ��K+��[Ag(NH3)2]+��Br����Cl�� |
| B���ں���Al3+��Cl������Һ�У�HCO3����I����NH4+��Mg2+ |
| C����c��H+��=1��10-13 mol��L-1����Һ�У�Na+��S2����SO32����NO3�� |
| D��������ΪKNO3��NaHSO4����Һ�У�Fe2+��Ca2+��Al3+��Cl�� |
�������ӷ���ʽ��ȷ����
| A���������ᷴӦ��2Fe +6H+ ==2Fe3++3H2�� |
| B��������ˮ��Ӧ��Cl2+H2O ="=" 2H++Cl+ClO�� |
| C������������������Һ��Ӧ��Ba2++OH��+H++SO42��=BaSO4��+ H2O |
| D����������ˮ��Ӧ��2Na +2H2O ==2Na++2OH��+H2�� |
����ʾ��ͼ���Ӧ�ķ�Ӧ�����ȷ����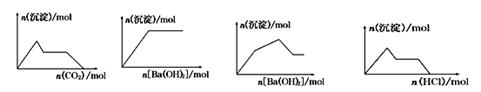
A B C D
| A����0��01 mol KOH��0��01 mol Ca(OH)2�Ļ����Һ�л���ͨ��CO2 |
| B��NaHSO4��Һ����μ���Ba(OH)2��Һ |
| C��KAl(SO4)2��Һ����μ���Ba(OH)2��Һ |
| D��NaAlO2��Һ����μ������� |
�����£������и�����Һ�У���ˮϡ��ʱc(OH��) / c(H+)ֵ�����������ܴ���������������ǣ� ��
�� K+��Al3+��NO3����AlO2�� �� K+��Fe3+��I����SO42��
�� Ba2+��I����NO3����Na+ �� Na+��Ca2+��Al3+�� Cl��
| A���٢� | B���٢� | C���ڢ� | D���ڢ� |
����˵����ȷ����
| A��CO2��ˮ��Һ�ܵ��磬����CO2�ǵ���ʣ�Һ�岻���磬����Һ���Ƿǵ���� |
| B��BaSO4������ˮ����ˮ��Һ�ĵ�����������������BaSO4��������� |
| C��ij���ʾ�������ֻ����һ��Ԫ�أ��������һ����һ�ֵ��� |
| D��ǿ�������Һ�ĵ���������һ�������������Һ�ĵ�������ǿ |
�������ӷ���ʽ��д��ȷ����
| A������ʯ��ˮ������С�մ���Һ��ϣ�Ca2+ +2OH- +2HCO3- = CaCO3��+ CO32- + 2H2O |
| B���Ȼ�����Һ�м�������İ�ˮ��Al3+ + 4NH3��H2O = Al(OH)4-��+ 4NH4+ |
| C������CO2ͨ�뱽������Һ�У�2C6H5O-+CO2+H2O=2C6H5OH+ CO32- |
| D����NH4HSO4ϡ��Һ����μ���Ba(OH)2ϡ��Һ��SO42-�պó�����ȫ��Ba2+ + 2OH- + NH4+ + H+ + SO4 2- = BaSO4��+ NH3��H2O + H2O |
�����������ڷǵ���ʵ���
| A�������� | B��CaCl2 | C��H2SO4 | D��SO2 |
����״̬�����ʣ����ܵ��������ڵ���ʵ���
| A��MgCl2���� | B��NaCl��Һ | C��Һ̬HCl | D�����ڵ�KOH |