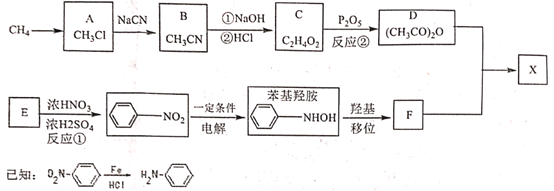
��Ŀ����
����Ŀ��1,2-����������һ�ֹ㷺ʹ�õ��л��ܼ����ϼ���Ҳ������������ֵ�Ѭ�������е�83.5�棬�۵㣭35�档ij�о���ѧϰС���ͬѧ������ͼ(����װ��ʡ��)װ���Ʊ�һ������1,2-�������飬�Ʊ�ԭ��Ϊ��C2H5OH![]() C2H4��CH2ClCH2Cl��װ��A�е�Ũ�����Ǵ�������ˮ�����Ҵ����ܶ�ԼΪ0.8g/mL ��
C2H4��CH2ClCH2Cl��װ��A�е�Ũ�����Ǵ�������ˮ�����Ҵ����ܶ�ԼΪ0.8g/mL ��

(1)�����Ʊ�ԭ������֪װ��A�л�ȱ�ٵ�һ��ʵ��������________��ʹ�������ܵ�Ŀ����__________________________��
(2)ʵ��ʱA��������ƿ�����д̼�����ζ�������������Ϊ���շ�Ӧ�����ɵ������壬��װ��B��Ӧ����________(����ĸ���)��
a��ˮ b��Ũ���� c������������Һ d������̼��������Һ
(3)D��a��c�������ܽ��������еij��Ȳ�ͬ�����ŵ���________________���Ե���b�Ľ�һ������������______________________��װ��E������������ƿ����Q�е�������______________��
(4)���õ�ag1,2-�������飬���Ҵ���������Ϊ_____________________________��
���𰸡��¶ȼ� ʹ�Ҵ��������������ԭ�ϵ������� c ������Cl2��C2H4��ֻ�Ϸ�Ӧ ��b���ݳ���Cl2���뵽NaOH��Һ�� ����ʳ��ˮ ![]()
��������
Aװ��Ϊ�Ҵ���Ũ�����ϼ�������ϩ������B��NaOH��Һ��ȥ������CO2��SO2����Cװ�û������D������E��������������ӳɷ�Ӧ����CH2ClCH2Cl������ag1,2-���������Ʒ��������۲μӷ�Ӧ���Ҵ��������ٽ��ʵ���ṩ���Ҵ����������Ҵ��������ʡ�
(1)�Ҵ���Ũ�����ϼ�����170��ʱ������ȥ��Ӧ������ϩ����װ��A�л�ȱ�ٵ�һ��ʵ���������¶ȼƣ��Ҵ��ӷ�����ʹ�������ܵ�Ŀ����ʹ�Ҵ��������������ԭ�ϵ������ʣ�
(2)��Ũ������ǿ�����ԣ��ڼ����������������Ҵ�����CO2��ͬʱŨ���ỹԭ����SO2��CO2��SO2�����������������NaOH��Һ��Ӧ�����κ�ˮ����Ϊ���շ�Ӧ�����ɵ�CO2��SO2Ӧѡ������������Һ���ʴ�Ϊc��
(3)��ϩ���������ܶȲ�ͬ����D��a��c�������ܽ��������еij��Ȳ�ͬ��Ŀ����������Cl2��C2H4��ֻ�Ϸ�Ӧ���������ж���Ϊ��ֹ��Ⱦ��������Ӧ��b���ݳ���Cl2���뵽NaOH��Һ�У�����������ˮ��Ϊʹװ��E�������ݳ���Q�е�����Ӧѡ��ʳ��ˮ��
(4) ag1,2-������������ʵ���Ϊ![]() =
=![]() mol����C2H5OH
mol����C2H5OH![]() C2H4��CH2ClCH2Cl��֪���۲μӷ�Ӧ����ϩ�����ʵ���Ϊ
C2H4��CH2ClCH2Cl��֪���۲μӷ�Ӧ����ϩ�����ʵ���Ϊ![]() mol�����Ҵ���������Ϊ
mol�����Ҵ���������Ϊ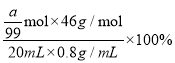 =
=![]() ��
��

 �����ܿ����ϵ�д�
�����ܿ����ϵ�д�