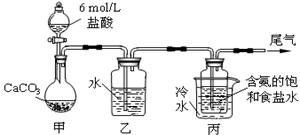
��Ŀ����
����һ��Ӧ�ù㷺�Ľ�������ҵ����Al2O3�ͱ���ʯ(Na3AlF6)������ڵ���Ƶá����������Ҫ�ɷ���Al2O3����������SiO2��Fe2O3��MgO����ҵ�ϴ�������������Al2O3����ȡ�����������£�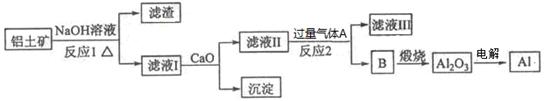
��ش��������⣺
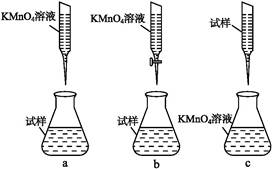
��1������NaOH��Һ��Ӧ�����ӷ���ʽΪ ��
��2��ͼ���漰������Һ������IJ����������ձ�����������_________��
��3����Ӧ1�漰�Ļ�ѧ����ʽ�� �� ����Һ���м���CaO���ɵij�����_ (�ѧʽ)��
��4������Һ����ͨ������AΪ �����ɳ���B�����ӷ���ʽΪ ��
��1��2Al+2H2O+2OH��=2AlO2��+3H2�� ��2��©��
��3��Al2O3+2NaOH=2NaAlO2+H2O SiO2+2NaOH=Na2SiO3+H2O CaSiO3
��4��CO2 AlO2��+2H2O+CO2=Al(OH)3��+HCO3��
���������������1������NaOH��Һ��Ӧ�����ӷ���ʽΪ2Al+2H2O+2OH��=2AlO2��+3H2��;��2��ͼ���漰������Һ������IJ����������ձ�����������©������3�������������������Ʒ�Ӧ���ɹ�������ˮ����Ӧ����ʽΪ��2NaOH+SiO2�TNa2SiO3+H2O�����������������Ʒ�Ӧ����ƫ��������ˮ����Ӧ����ʽΪ��2NaOH+Al2O3�T2NaAlO2+H2O����Һ���к��й����ơ�ƫ�����ƣ�����CaO�������������ƣ���4���ɹ������̿�֪��BΪ����������������AΪ������̼����Һ����Ҫ��ƫ�����ƣ�ƫ��������Һͨ�������̼����������������̼�����ƣ������ӷ�Ӧ����ʽΪ��AlO2��+2H2O+CO2=Al(OH)3��+HCO3����
���㣺���ʷ�����ᴿ�ķ����ͻ��������ۺ�Ӧ��

 ����ȫ���ִʾ��ƪ��ϵ�д�
����ȫ���ִʾ��ƪ��ϵ�д� �����߿����ϵ�д�
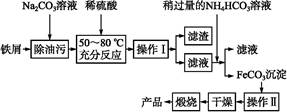
�����߿����ϵ�д��ú���Al2O3������Fe2O3��SiO2���������Ʊ���ˮ������Һ��ۺ����������������������£����ֲ����������ԣ���
I�����������м������H2SO4���ȡ����衢���ˡ�
II������Һ�м���һ������FeSO4��7H2O��˫��ˮ��
III������Һ�м���Ca(OH)2���壬������Һ��pHԼΪ1�����ˡ�
IV�������ȶ��������ȣ��õ���Ʒ��
��1��Fe2O3��H2SO4��Ӧ�����ӷ���ʽ��___________��
��2������I�й��˵õ��������ɷ���________���ѧʽ����
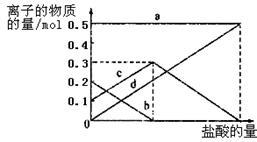
��3������I ��H2SO4��Ũ���뷴Ӧ�¶Ȼ�Ӱ���������Ľ����ʡ�������ͼ����������I ��H2SO4Ũ�ȵ����˷�Χ��__________����Ӧ�������¶���_________��


��4������II������n(Fe3+)�����ӷ���ʽ��_________��
��5������III�õ���ʽ��������[AlFe(OH)n(SO4)m]����Һ������II��Ӧ����n(Fe3+)��
n(Al3+)�sn(Fe3+)= ��
��6���о�������Һ��ۺ����������Ĵ���Խ�ߣ���ˮЧ��Խ�á���֪��
һЩ������20��ʱ���ܽ��
| ���� | Ca(OH)2 | CaSO4 | Na2SO4 |
| �ܽ��/g | 0.153 | 0.258 | 19.5 |
��ϱ������ݣ����Ͳ���III��ʹ��Ca(OH)2������NaOH��ԭ��__________��
��7��������Ҳ������ұ������Al���Խ���Al��������ϡ���������Һ��ͨ������ʹ����Al�ı����������ܼ�Ӳ������Ĥ����缫��Ӧʽ��_________��


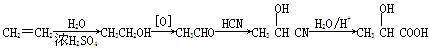
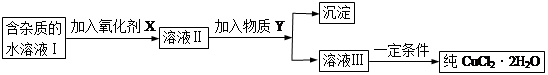

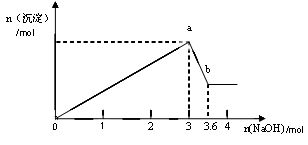
 b�仯���̵����ӷ���ʽ ��
b�仯���̵����ӷ���ʽ ��
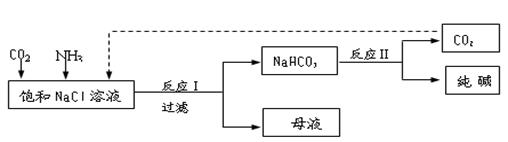 k+s-5#
k+s-5# NaHCO3��+NH4Cl������ĸҺ�����ַ������£�
NaHCO3��+NH4Cl������ĸҺ�����ַ������£�