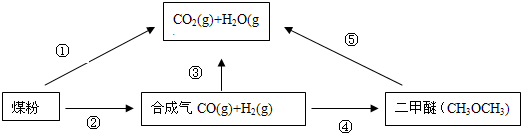
��Ŀ����
ú��������Һ���Ǹ�Ч����������ú����Ҫ�������÷����Ļ�ѧ��Ӧ��Ҫ��̼��ˮ������Ӧ����A��B�Ļ�����壬Ȼ�����������Ǻϳɶ����л��ͼ1-5��ijЩ���ʵĺϳ�·�ߡ�A��B����ͬ����������C��D��E������F��һ����ɫ���д̼�����ζ�����壬�Ǽ�ͥװ�곣����Ⱦ�C�ɷֱ����ƺ�������Һ��Ӧ��
ͼ1-5
��ش�
(1)���ⶨ���л���J��̼����Ԫ��������������Ϊ40.68����5.08��������Ϊ��Ԫ�ء�J��H2������ܶ���59��д��J�ķ���ʽ��_______________________��
(2)д���������ʵĽṹ��ʽ��E_________________________C_____________________��
(3)��������������Һ�IJ�����_____________________________________________��
(4)д�����л�ѧ��Ӧ����ʽ����ע����Ӧ���͡�
G+I![]() J__________________________________�����ͣ�___________________��
J__________________________________�����ͣ�___________________��
H![]() I__________________________________�����ͣ�___________________��
I__________________________________�����ͣ�___________________��
(5)д��һ���������࣬����J��Ϊͬ���칹��Ľṹ��ʽ��_____________________________��
(1)C4H6O4 (2)E��CH3OH C��HOCH2CHO
(3)һ������AgNO3��Һ�еμ�ϡ��ˮ��ֱ�����ɵİ�ɫ����ǡ����ʧΪֹ
(4)G+I![]() J��2HCOOH+HOCH2CH2OH
J��2HCOOH+HOCH2CH2OH![]() HCOOCH2CH2OCHO+2H2O
HCOOCH2CH2OCHO+2H2O
������Ӧ��ȡ����Ӧ
H![]() I��BrCH2CH2Br
I��BrCH2CH2Br![]() HOCH2CH2OH+2HBrˮ���ȡ����Ӧ
HOCH2CH2OH+2HBrˮ���ȡ����Ӧ
(5) ![]() ��
��
��ʾ�����ݿ�ͼ�С�E![]() F
F![]() G ���ɲ²�E�Ǵ���F��ȩ��G���ᣬ�ٽ�ϡ�F��һ����ɫ���д̼�����ζ�����塱��ȷ��F�Ǽ�ȩ������ȷ��E�Ǽ״���G�Ǽ��ᡣ
G ���ɲ²�E�Ǵ���F��ȩ��G���ᣬ�ٽ�ϡ�F��һ����ɫ���д̼�����ζ�����塱��ȷ��F�Ǽ�ȩ������ȷ��E�Ǽ״���G�Ǽ��ᡣ
���ݡ�J��̼����Ԫ��������������Ϊ40.68����5.08��������Ϊ��Ԫ�ء��͡�J��H2������ܶ���
�ۺ��������ۿɽ�һ���ƶ�J���Ҷ�������һ�����жϳ�H��1��2-�������飬D����ϩ��C�Ľṹ��ʽΪ ![]() ��
��
���������漰�������ƶϡ��������㣬����J��һ�ֶ�Ԫ��������İ���ȱȽϸߣ������ѶȱȽϴ����ۺ��������������Դ���������

| A����ѧ���ʷ�����Ӧ���Ȼ������ɷ�Ӧ�������������������� | B����ͨ����ú����ȴ�ʩ���úȼ��Ч�� | C�����������п�����ɷ���Σ������Ӧ�����䷢չ | D��ú��������Һ���Ǹ�Ч����������ú̿����Ҫ;�� |