��Ŀ����
��12�֣���ͼ��ʾװ�ÿ�������ȡ�۲�Fe(OH)2�ڿ����б�����ʱ����ɫ�仯��ʵ��ʱ����ʹ����м��6 mol/L����������Լ���ѡ��
��д���пհף�
��1��B��ʢ��һ������NaOH��Һ��A�� ӦԤ�ȼ�����Լ���________��A�з�Ӧ�����ӷ���ʽΪ____________________________________________________��
ӦԤ�ȼ�����Լ���________��A�з�Ӧ�����ӷ���ʽΪ____________________________________________________��
��2��ʵ�鿪ʼʱӦ�Ƚ�����D_____����Ŀ����____����C���ռ�����������Ҫ��_____________________
��3����������Fe(OH)2�IJ�������______________________________________________
��4���ε�װ��B�е�������ʹ�������룬д���йط�Ӧ�Ļ�ѧ����ʽ________________
��1����м Fe+2H+==Fe2++H2��
��2�� �� �ų�B�еĿ��� ����
��3���رջ���D��ʹA�е�FeSO4��Һѹ��Bƿ����NaOH��Ӧ���Ӷ��Ƶ�Fe(OH)2
��4��4Fe(OH)2+O2+2H2O====4Fe(OH)3
����

��ϰ��ϵ�д�
�����Ŀ
 ʵ���ҿ�����ͼ��ʾ��װ����ȡ���壬�����й�˵����ȷ���ǣ�������
ʵ���ҿ�����ͼ��ʾ��װ����ȡ���壬�����й�˵����ȷ���ǣ�������| A��ʵ���Ҳ���������װ������ȡ���� | B������װ�ÿ��Բ��ü����Թ������װ�õ������� | C��ʳ�θ�Ũ���ᷴӦ���Ȼ����ʵ����������װ�� | D����������װ����ȡ��Ȳ����������ͣ |
 ��ͼ��ʾװ�ÿ����ڶ���ʵ�飮
��ͼ��ʾװ�ÿ����ڶ���ʵ�飮
 ��˵�����¾��ϴ��ر��㡱����ԭ���Ǿ��ڴ������������������ζ��������������ʵ����������Ҳ��������ͼ��ʾ��װ����ģ��ù��̣���ش��������⣺
��˵�����¾��ϴ��ر��㡱����ԭ���Ǿ��ڴ������������������ζ��������������ʵ����������Ҳ��������ͼ��ʾ��װ����ģ��ù��̣���ش��������⣺
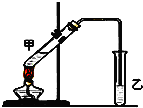 ��˵�����¾��ϴ��ر��㡱����ԭ���Ǿ��ڴ������������������ζ��������������ʵ����������Ҳ��������ͼ��ʾ��װ����ģ��ù��̣���ش��������⣺
��˵�����¾��ϴ��ر��㡱����ԭ���Ǿ��ڴ������������������ζ��������������ʵ����������Ҳ��������ͼ��ʾ��װ����ģ��ù��̣���ش��������⣺ ����ͼ��ʾװ�ÿ�������ȡ
����ͼ��ʾװ�ÿ�������ȡ ���Ƚ�SԪ����BrԪ�طǽ����Ե�ǿ����
���Ƚ�SԪ����BrԪ�طǽ����Ե�ǿ����
 ���ϣ�
���ϣ� ��Zn��Ӧ������Ũ
��Zn��Ӧ������Ũ