��Ŀ����
��4molSO2��2molO2����2L���ܱ������У���һ�������·�����Ӧ����2s����SO3��Ũ��Ϊ0.6mol��L��1������˵������ȷ���ǣ� ��
����O2��ʾ�ķ�Ӧ��ƽ������Ϊ0.6mol��L��1��s��1
����SO2��ʾ�ķ�Ӧ��ƽ������Ϊ0.3mol��L��1��s��1
��2sʱSO2��ת����Ϊ15%
��2sʱO2��Ũ��Ϊ0.7mol��L��1
����O2��ʾ�ķ�Ӧ��ƽ������Ϊ0.6mol��L��1��s��1
����SO2��ʾ�ķ�Ӧ��ƽ������Ϊ0.3mol��L��1��s��1
��2sʱSO2��ת����Ϊ15%
��2sʱO2��Ũ��Ϊ0.7mol��L��1
| A���٢� | B���٢� | C���ڢ� | D���ڢ� |
D
2SO2+O2 2SO3
2SO3
��ʼŨ�� 2 1 0
ת��Ũ�� 0.6 0.3 0.6
ƽ��Ũ�� 1.4 0.7 0.6
����O2��ʾ�ķ�Ӧ��ƽ������Ϊ0.15mol��L��1��s��1
����SO2��ʾ�ķ�Ӧ��ƽ������Ϊ0.3mol��L��1��s��1
��2sʱSO2��ת����Ϊ30%
��2sʱO2��Ũ��Ϊ0.7mol��L��1
�ڡ�����ȷ����ѡD
 2SO3
2SO3��ʼŨ�� 2 1 0
ת��Ũ�� 0.6 0.3 0.6
ƽ��Ũ�� 1.4 0.7 0.6
����O2��ʾ�ķ�Ӧ��ƽ������Ϊ0.15mol��L��1��s��1
����SO2��ʾ�ķ�Ӧ��ƽ������Ϊ0.3mol��L��1��s��1
��2sʱSO2��ת����Ϊ30%
��2sʱO2��Ũ��Ϊ0.7mol��L��1
�ڡ�����ȷ����ѡD

��ϰ��ϵ�д�
�����Ŀ

 4Y(g)��Z(g)����H<0����ij�¶�ʱ
4Y(g)��Z(g)����H<0����ij�¶�ʱ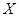 ��Ũ����ʱ��仯�����ߣ�
��Ũ����ʱ��仯�����ߣ�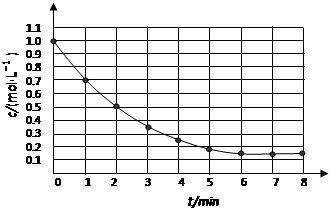
 ��Ӧ����ֹ��
��Ӧ����ֹ�� ���ڿ�ʼʱ��Ӧ�ٶ�
���ڿ�ʼʱ��Ӧ�ٶ�

 CH3OH(g)+H2O(g);�SH<0��Ϊ��̽���䷴Ӧԭ����������ʵ�飬��2L�ܱ�������250�������£����n(CO2)��ʱ��ı仯������±���
CH3OH(g)+H2O(g);�SH<0��Ϊ��̽���䷴Ӧԭ����������ʵ�飬��2L�ܱ�������250�������£����n(CO2)��ʱ��ı仯������±��� 2NH3(g)�����2min��N2��Ũ����6mol/L��С��2mol/L����ô��N2Ũ�ȱ仯����ʾ�ĸ÷�Ӧ����2min�ڵķ�Ӧ����Ϊ
2NH3(g)�����2min��N2��Ũ����6mol/L��С��2mol/L����ô��N2Ũ�ȱ仯����ʾ�ĸ÷�Ӧ����2min�ڵķ�Ӧ����Ϊ